Page 505 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 505
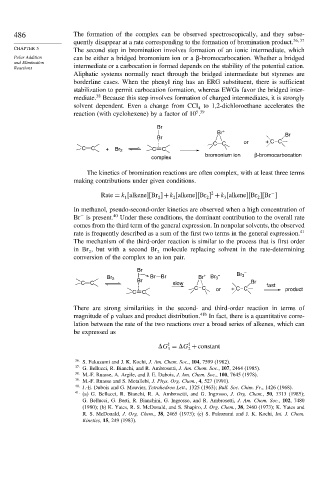
486 The formation of the complex can be observed spectroscopically, and they subse-
quently disappear at a rate corresponding to the formation of bromination product. 36 37
CHAPTER 5 The second step in bromination involves formation of an ionic intermediate, which
Polar Addition can be either a bridged bromonium ion or a -bromocarbocation. Whether a bridged
and Elimination
Reactions intermediate or a carbocation is formed depends on the stability of the potential cation.
Aliphatic systems normally react through the bridged intermediate but styrenes are
borderline cases. When the phenyl ring has an ERG substituent, there is sufficient
stabilization to permit carbocation formation, whereas EWGs favor the bridged inter-
38
mediate. Because this step involves formation of charged intermediates, it is strongly
solvent dependent. Even a change from CCl to 1,2-dichloroethane accelerates the
4
5 39
reaction (with cyclohexene) by a factor of 10 .
Br
Br +
Br Br
CC or + CC
C C + Br 2 C C
bromonium ion β-bromocarbocation
complex
The kinetics of bromination reactions are often complex, with at least three terms
making contributions under given conditions.
2
−
Rate = k alkene Br +k alkene Br +k alkene Br Br
3
2
1
2
2
2
In methanol, pseudo-second-order kinetics are observed when a high concentration of
40
−
Br is present. Under these conditions, the dominant contribution to the overall rate
comes from the third term of the general expression. In nonpolar solvents, the observed
rate is frequently described as a sum of the first two terms in the general expression. 41
The mechanism of the third-order reaction is similar to the process that is first order
in Br , but with a second Br molecule replacing solvent in the rate-determining
2
2
conversion of the complex to an ion pair.
Br –
Br Br Br + Br 3 – Br 3
Br 2 Br
C C slow Br fast
CC or + CC product
C C
There are strong similarities in the second- and third-order reaction in terms of
magnitude of
values and product distribution. 41b In fact, there is a quantitative corre-
lation between the rate of the two reactions over a broad series of alkenes, which can
be expressed as
‡
‡
G = G +constant
3
2
36
S. Fukuzumi and J. K. Kochi, J. Am. Chem. Soc., 104, 7599 (1982).
37
G. Bellucci, R. Bianchi, and R. Ambrosetti, J. Am. Chem. Soc., 107, 2464 (1985).
38 M.-F. Ruasse, A. Argile, and J. E. Dubois, J. Am. Chem. Soc., 100, 7645 (1978).
39
M.-F. Ruasse and S. Motallebi, J. Phys. Org. Chem., 4, 527 (1991).
40 J.-E. Dubois and G. Mouvier, Tetrahedron Lett., 1325 (1963); Bull. Soc. Chim. Fr., 1426 (1968).
41
(a) G. Bellucci, R. Bianchi, R. A. Ambrosetti, and G. Ingrosso, J. Org. Chem., 50, 3313 (1985);
G. Bellucci, G. Berti, R. Bianchini, G. Ingrosso, and R. Ambrosetti, J. Am. Chem. Soc., 102, 7480
(1980); (b) K. Yates, R. S. McDonald, and S. Shapiro, J. Org. Chem., 38, 2460 (1973); K. Yates and
R. S. McDonald, J. Org. Chem., 38, 2465 (1973); (c) S. Fukuzumi and J. K. Kochi, Int. J. Chem.
Kinetics, 15, 249 (1983).