Page 491 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 491
472 CH 3 CH 2 + + CH 3 CH 2 X CH 3 CH 3 + CH 3 CH X
+
+
CHAPTER 4 CH 2 CH + + CH 2 CHX CH 2 CH 2 + CH 2 C X
Nucleophilic Substitution
a. The stabilization of vinyl cations tends to be somewhat larger than for the
corresponding ethyl cation.
b. F, OH, and NH provide less stabilization of vinyl cations than of ethyl
2
cations.
c. CF and CN are less destabilizing of vinyl cations than of ethyl cations.
3
d. What factors dominate the effect of the CH −X substituents?
2
e. Compare the -donor and polar effects of the OH, NH , and SH substituents.
2
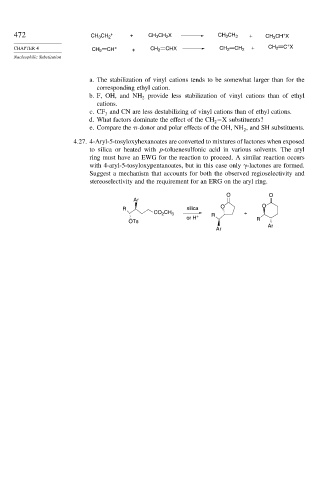
4.27. 4-Aryl-5-tosyloxyhexanoates are converted to mixtures of lactones when exposed
to silica or heated with p-toluenesulfonic acid in various solvents. The aryl
ring must have an EWG for the reaction to proceed. A similar reaction occurs
with 4-aryl-5-tosyloxypentanoates, but in this case only -lactones are formed.
Suggest a mechanism that accounts for both the observed regioselectivity and
stereoselectivity and the requirement for an ERG on the aryl ring.
O O
Ar
R silica O O
CO CH 3 R +
2
or H + R
OTs
Ar
Ar