Page 486 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 486
467
5
5
5
−1
5
−1
s
Substituent 10 k 1 s 10 k 2 M −1 −1 Substituent 10 k 1 s 10 k 2 M −1 −1
s
PROBLEMS
4-CH 3 O 1660 2820 2-Naphthyl 0 28 11 6
4-CH 3 S 103 215 3-CH 3 0 055 7 29
4-C 6 H 5 O 41 5 119 H 0 032 5 54
3-Cl-4-CH 3 O 21 2 79 0 4-Cl 4 37
2-Fluorenyl 18 3 59 5 3-Cl 2 085
8 56 41 1 1 77
3,4,5-tri-CH 3 3-CF 3
3 67 28 3 1 21
3,4-di-CH 3 3-NO 2
1 46 19 2 1 19
4-CH 3 4-NO 2
4- CH 3 3 C 0 82 15 2 3,5-di-CF 3 0 651
–1
p-MeO
p-MeO
–2
p-MeS
p-PhO
–3 p-MeS p-MeO-m-Cl
2-Fl
3,4,5-Me
p-PhO 3,4-Me 3
p-MeO-m-Cl p-Me 2
p-t-Bu
2-Fl 2-Naph
log k –4 3,4,5-Me 3 m-Me p-Cl
H
3,4-Me
2 m-Cl
m-CF 3
p-Me p-NO
2
–5 p-t-Bu m-NO 2
3,5-(CF ) 3 2
2-Naph
log k = –5.0 σ –
–6 1
m-Me
H
–7
–1.2 – 0.8 – 0.4 0.0 0.4 0.8 1.2
–
σ (r = 1.15)
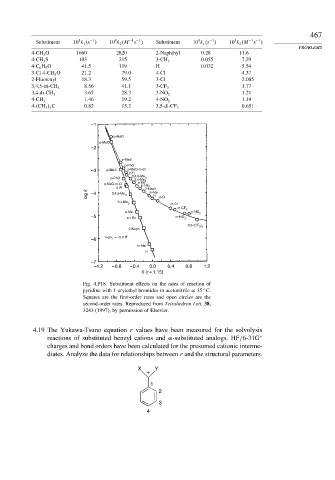
Fig. 4.P18. Substituent effects on the rates of reaction of
pyridine with 1-arylethyl bromides in acetonitrile at 35 C.
Squares are the first-order rates and open circles are the
second-order rates. Reproduced from Tetrahedron Lett. 38,
3243 (1997), by permission of Elsevier.
4.19 The Yukawa-Tsuno equation r values have been measured for the solvolysis
reactions of substituted benzyl cations and
-substituted analogs. HF/6-31G ∗
charges and bond orders have been calculated for the presumed cationic interme-
diates. Analyze the data for relationships between r and the structural parameters.
X Y
+
1
2
3
4