Page 482 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 482
18
18
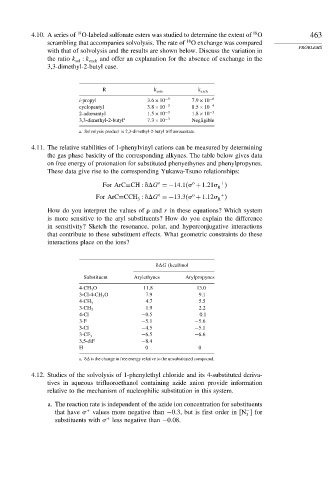
4.10. A series of O-labeled sulfonate esters was studied to determine the extent of O 463
18
scrambling that accompanies solvolysis. The rate of O exchange was compared
PROBLEMS
with that of solvolysis and the results are shown below. Discuss the variation in
the ratio k sol k exch and offer an explanation for the absence of exchange in the
3,3-dimethyl-2-butyl case.
R k solv k exch
i-propyl 3 6×10 −5 7 9×10 −6
cyclopentyl 3 8×10 −3 8 5×10 −4
2-adamantyl 1 5×10 −3 1 8×10 −3
3,3-dimethyl-2-butyl a 7 3×10 −3 Negligible
a. Solvolysis product is 2,3-dimethyl-2-butyl trifluoroacetate.
4.11. The relative stabilities of 1-phenylvinyl cations can be measured by determining
the gas phase basicity of the corresponding alkynes. The table below gives data
on free energy of protonation for substituted phenyethynes and phenylpropynes.
These data give rise to the corresponding Yukawa-Tsuno relationships:
o
o
For ArC≡CH G =−14 1 +1 21 +
R
o
o
For ArC≡CCH G =−13 3 +1 12 +
3 R
How do you interpret the values of
and r in these equations? Which system
is more sensitive to the aryl substituents? How do you explain the difference
in sensitivity? Sketch the resonance, polar, and hyperconjugative interactions
that contribute to these substituent effects. What geometric constraints do these
interactions place on the ions?
G (kcal/mol
Substituent Arylethynes Arylpropynes
4-CH 3 O 11.8 13.0
3-Cl-4-CH 3 O 7.9 9.1
4.7 5.5
4-CH 3
1.9 2.2
3-CH 3
4-Cl −0 5 0.1
3-F −5 1 −5 6
3-Cl −4 5 −5 1
−6 5 −6 6
3-CF 3
3,5-diF −8 4
H 0 0
a. is the change in free energy relative to the unsubstituted compound.
4.12. Studies of the solvolysis of 1-phenylethyl chloride and its 4-substituted deriva-
tives in aqueous trifluoroethanol containing azide anion provide information
relative to the mechanism of nucleophilic substitution in this system.
a. The reaction rate is independent of the azide ion concentration for substituents
that have values more negative than −0 3, but is first order in N for
+
−
3
substituents with less negative than −0 08.
+