Page 483 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 483
464 b. When other good nucleophiles, e.g., C H SH, are present, they can compete
7
3
−
with azide ion. The reactants that are zero order in N show little selectivity
3
CHAPTER 4
among competing nucleophiles.
Nucleophilic Substitution c. For reactants that solvolyze at rates independent of N , the ratio of
−
3
1-arylethyl azide to 1-arylethanol in the product increases as + of the
substituent becomes more negative.
d. The major product in reactions that are first order in N are 1-arylethyl
−
3
azides.
Consider these results in relation to the mechanism outlined in Figure 4.6
(p. 400). On the basis of the data given above, delineate the types of 1-arylethyl
chlorides that react with azide ion according to those mechanistic types.
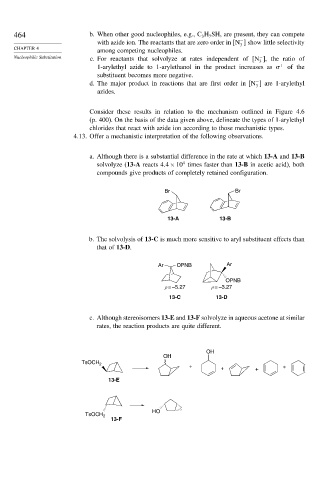
4.13. Offer a mechanistic interpretation of the following observations.
a. Although there is a substantial difference in the rate at which 13-A and 13-B
4
solvolyze (13-A reacts 4 4×10 times faster than 13-B in acetic acid), both
compounds give products of completely retained configuration.
Br Br
13-A 13-B
b. The solvolysis of 13-C is much more sensitive to aryl substituent effects than
that of 13-D.
Ar OPNB Ar
OPNB
ρ = –5.27 ρ = –3.27
13-C 13-D
c. Although stereoisomers 13-E and 13-F solvolyze in aqueous acetone at similar
rates, the reaction products are quite different.
OH
OH
TsOCH 2
+ + + +
13-E
HO
TsOCH 2
13-F