Page 484 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 484
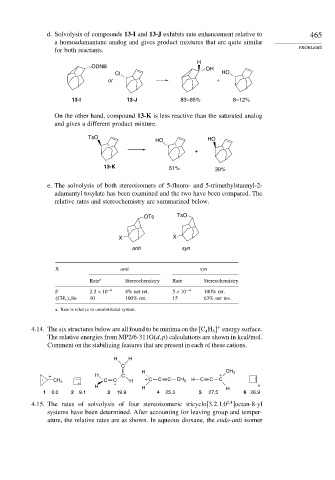
d. Solvolysis of compounds 13-I and 13-J exhibits rate enhancement relative to 465
a homoadamantane analog and gives product mixtures that are quite similar
for both reactants. PROBLEMS
H
ODNB
OH
Cl HO
or +
13-I 13-J 83 – 85% 8 – 12%
On the other hand, compound 13-K is less reactive than the saturated analog
and gives a different product mixture.
TsO HO
HO
+
13-K 51% 39%
e. The solvolysis of both stereoisomers of 5-fluoro- and 5-trimethylstannyl-2-
adamantyl tosylate has been examined and the two have been compared. The
relative rates and stereochemistry are summarized below.
OTs TsO
X X
anti syn
X anti syn
Rate a Stereochemistry Rate Stereochemistry
F 2 5×10 −6 4% net ret. 5×10 −4 100% ret.
CH 3 3 Sn 10 100% ret. 15 63% net inv.
a. Rate is relative to unsubstituted system.
+
4.14. The six structures below are all found to be minima on the C H energy surface.
4
5
The relative energies from MP2/6-311G(d,p) calculations are shown in kcal/mol.
Comment on the stabilizing features that are present in each of these cations.
H H
C
H CH 3
+ H C +
C C + C C C CH 3 H C C C
CH 3 H
+ +
H H H +
1 0.0 2 9.1 3 19.9 4 25.3 5 27.5 6 26.9
2 4
4.15. The rates of solvolysis of four stereoisomeric tricyclo[3.2.1.0 ]octan-8-yl
systems have been determined. After accounting for leaving group and temper-
ature, the relative rates are as shown. In aqueous dioxane, the endo-anti isomer