Page 479 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 479
−9 −1
−9 −1
−5 −1
460 C H 1 1 × 10 s i–C H 5 0 × 10 s t–C H 3 4 × 10 s
4
7
3
2
5
9
−9 −1
CH CCH 1 5×10 s .
2
3 3
CHAPTER 4
Nucleophilic Substitution
R
OPNB
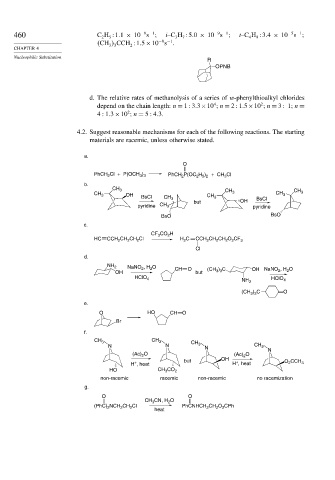
d. The relative rates of methanolysis of a series of w-phenylthioalkyl chlorides
4 2
depend on the chain length: n = 1 3 3×10 n = 2 1 5×10 n = 3 1 n =
2
4 1 3×10 n = 5 4 3.
4.2. Suggest reasonable mechanisms for each of the following reactions. The starting
materials are racemic, unless otherwise stated.
a.
O
PhCH Cl + P(OCH ) PhCH 2 P(OC 2 H 5 ) 2 + CH 3 Cl
3 3
2
b.
CH 3
CH 3 CH 3
CH 3 OH BsCl CH 3 CH 3 CH 3
but OH BsCl
pyridine CH 3 pyridine
BsO BsO
c.
CF 3 CO 2 H
HC CCH 2 CH 2 CH 2 Cl H 2 C CCH 2 CH 2 CH 2 O 2 CF 3
Cl
d.
NH 2 NaNO 2 , H 2 O CH O (CH 3 ) 3 C OH NaNO 2 , H 2 O
OH but
HClO 4 HClO 4
NH 2
(CH 3 ) 3 C O
e.
O HO CH O
Br
f.
CH 3 CH 3 CH 3
N N N CH 3
(Ac) 2 O (Ac) 2 O N
but OH + O 2 CCH 3
+
H , heat H , heat
HO CH 3 CO 2
non-racemic racemic non-racemic no racemization
g.
O O
CH 3 CN, H 2 O
(PhC) 2 NCH 2 CH 2 Cl PhCNHCH 2 CH 2 O 2 CPh
heat