Page 478 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 478
459
C
C C C C 1 C PROBLEMS
C 1 C 2 2
H
H 2 2 H 1
H 1
O
O 2 Si 2 O
Si O 1 1 Si
Si
Al
Al
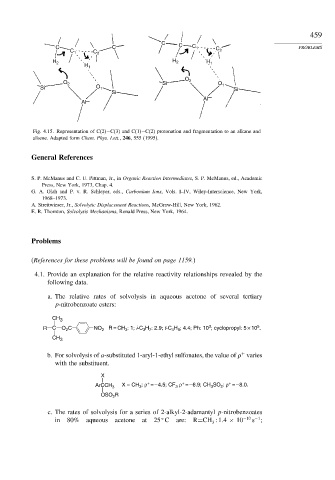
Fig. 4.15. Representation of C(2)−C(3) and C(1)−C(2) protonation and fragmentation to an alkane and
alkene. Adapted form Chem. Phys. Lett., 246, 555 (1995).
General References
S. P. McManus and C. U. Pittman, Jr., in Organic Reaction Intermediates, S. P. McManus, ed., Academic
Press, New York, 1973, Chap. 4.
G. A. Olah and P. v. R. Schleyer, eds., Carbonium Ions, Vols. I–IV, Wiley-Interscience, New York,
1968–1973.
A. Streitwieser, Jr., Solvolytic Displacement Reactions, McGraw-Hill, New York, 1962.
E. R. Thornton, Solvolysis Mechanisms, Ronald Press, New York, 1964.
Problems
(References for these problems will be found on page 1159.)
4.1. Provide an explanation for the relative reactivity relationships revealed by the
following data.
a. The relative rates of solvolysis in aqueous acetone of several tertiary
p-nitrobenzoate esters:
CH 3
5
3
R C O C NO 2 R = CH : 1; i-C H : 2.9; t-C H ; 4.4; Ph: 10 ; cyclopropyl: 5 × 10 .
3 7
4 9
3
2
CH 3
b. For solvolysis of a-substituted 1-aryl-1-ethyl sulfonates, the value of varies
+
with the substituent.
X
+
+
+
ArCCH 3 X = CH 3 : ρ = – 4.5; CF ρ = – 6.9; CH SO : ρ = – 8.0.
2
3
3
OSO 2 R
c. The rates of solvolysis for a series of 2-alkyl-2-adamantyl p-nitrobenzoates
s ;
in 80% aqueous acetone at 25 C are: R=CH 1 4 × 10 −10 −1
3