Page 480 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 480
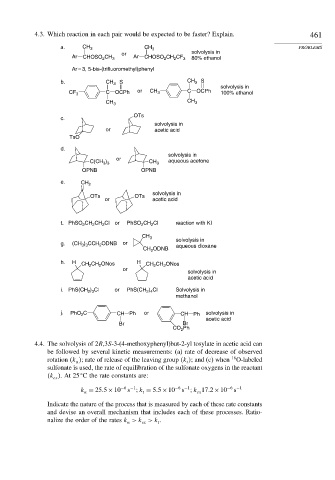
4.3. Which reaction in each pair would be expected to be faster? Explain. 461
a. CH 3 CH 3 PROBLEMS
or solvolysis in
Ar CHOSO 2 CH 3 Ar CHOSO CH CF 3 80% ethanol
2
2
Ar = 3, 5-bis-(trifluoromethyl)phenyl
b. CH 3 S CH 3 S
solvolysis in
CF 3 C OCPh or CH 3 C OCPh 100% ethanol
CH 3 CH 3
OTs
c.
solvolysis in
or acetic acid
TsO
d.
solvolysis in
or
)
C(CH 3 3 CH 3 aqueous acetone
OPNB OPNB
e. CH 2
solvolysis in
OTs OTs
or acetic acid
f. PhSO CH CH Cl or PhSO CH Cl reaction with KI
2
2
2
2
2
CH 3 solvolysis in
g. (CH ) CCH ODNB or aqueous dioxane
2
3 3
CH ODNB
2
h. H CH CH ONos H CH 2 CH ONos
2
2
2
or solvolysis in
acetic acid
i. PhS(CH ) Cl or PhS(CH ) Cl Solvolysis in
2 4
2 3
methanol
j. PhO 2 C CH Ph or CH Ph solvolysis in
acetic acid
Br Br
Ph
CO 2
4.4. The solvolysis of 2R,3S-3-(4-methoxyphenyl)but-2-yl tosylate in acetic acid can
be followed by several kinetic measurements: (a) rate of decrease of observed
18
rotation k ; rate of release of the leaving group k ; and (c) when O-labeled
t
sulfonate is used, the rate of equilibration of the sulfonate oxygens in the reactant
k .At25 C the rate constants are:
ex
s k 17 2×10
s
s k = 5 5×10
k = 25 5×10 −6 −1 t −6 −1 ex −6 −1
Indicate the nature of the process that is measured by each of these rate constants
and devise an overall mechanism that includes each of these processes. Ratio-
nalize the order of the rates k >k >k .
ex
t