Page 485 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 485
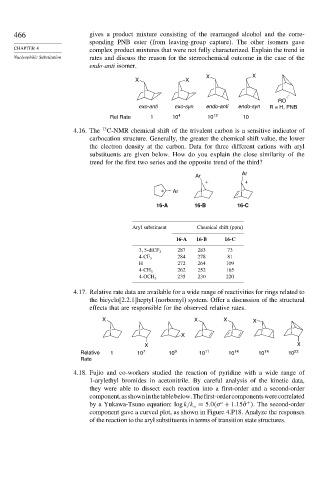
466 gives a product mixture consisting of the rearranged alcohol and the corre-
sponding PNB ester (from leaving-group capture). The other isomers gave
CHAPTER 4 complex product mixtures that were not fully characterized. Explain the trend in
Nucleophilic Substitution rates and discuss the reason for the stereochemical outcome in the case of the
endo-anti isomer.
X X
X X
RO
exo-anti exo-syn endo-anti endo-syn R = H, PNB
Rel Rate 1 10 4 10 12 10
4.16. The 13 C-NMR chemical shift of the trivalent carbon is a sensitive indicator of
carbocation structure. Generally, the greater the chemical shift value, the lower
the electron density at the carbon. Data for three different cations with aryl
substituents are given below. How do you explain the close similarity of the
trend for the first two series and the opposite trend of the third?
Ar
Ar
+ +
+ Ar
16-A 16-B 16-C
Aryl substituent Chemical shift (ppm)
16-A 16-B 16-C
287 283 73
3 5-diCF 3
284 278 81
4-CF 3
H 272 264 109
262 252 165
4-CH 3
235 230 220
4-OCH 3
4.17. Relative rate data are available for a wide range of reactivities for rings related to
the bicyclo[2.2.1]heptyl (norbornyl) system. Offer a discussion of the structural
effects that are responsible for the observed relative rates.
X X X X
X
X X
Relative 1 10 7 10 9 10 11 10 14 10 14 10 23
Rate
4.18. Fujio and co-workers studied the reaction of pyridine with a wide range of
1-arylethyl bromides in acetonitrile. By careful analysis of the kinetic data,
they were able to dissect each reaction into a first-order and a second-order
component,asshowninthetablebelow.Thefirst-ordercomponentswerecorrelated
o
by a Yukawa-Tsuno equation: logk/k = 5 0 + 1 15¯ . The second-order
+
o
component gave a curved plot, as shown in Figure 4.P18. Analyze the responses
of the reaction to the aryl substituents in terms of transition state structures.