Page 487 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 487
468
X,Y H,H CH 3 H CH 3 CH 3 CF 3 H
CHAPTER 4 r 1.28 1.15 1.00 1.51
Nucleophilic Substitution Mulliken
Charge
C(1) −0 0024 −0 050 −0 068 −0 211
C(2) +0 189 +0 164 +0 140 +0 053
C(3) +0 051 +0 046 +0 043 +0 053
C(4) +0 213 +0 190 +0 171 +0 233
Bond Order
C(1)−C(7) 1.584 1.465 1.363 1.622
C(1)−C(2) 1.158 1.193 1.225 1.134
C(2)−C(3) 1.167 1.543 1.524 1.585
C(3)−C(4) 1.343 1.361 1.375 1.329
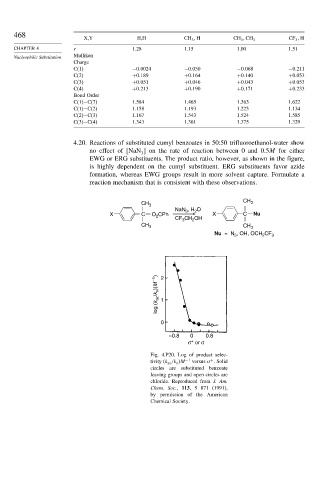
4.20. Reactions of substituted cumyl benzoates in 50:50 trifluoroethanol-water show
no effect of NaN on the rate of reaction between 0 and 0.5M for either
3
EWG or ERG substituents. The product ratio, however, as shown in the figure,
is highly dependent on the cumyl substituent. ERG substituents favor azide
formation, whereas EWG groups result in more solvent capture. Formulate a
reaction mechanism that is consistent with these observations.
CH
CH 3 3
NaN , H O
3
2
X C O CPh X C Nu
2
2
CF 3 CH OH
CH
CH 3 3
Nu = N , OH, OCH CF 3
2
3
log (k az /k s )(M –1 ) 2 1
0
–0.8 0 0.8
σ or σ
+
Fig. 4.P20. Log of product selec-
+
tivity (k az /k s M −1 versus . Solid
circles are substituted benzoate
leaving groups and open circles are
chloride. Reproduced from J. Am.
Chem. Soc., 113, 5 871 (1991),
by permission of the American
Chemical Society.