Page 561 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 561
542 This scheme shows an alkyne-bromine complex as an intermediate in all alkyne
brominations, which is analogous to the case of alkenes. The complex may dissociate
CHAPTER 5 to a vinyl cation when the cation is sufficiently stable, as is the case when there is
Polar Addition an aryl substituent. It may collapse to a bridged bromonium ion or undergo reaction
and Elimination
Reactions with a nucleophile. The latter is the dominant reaction for alkyl-substituted alkynes
and leads to stereospecific anti addition. Reactions proceeding through vinyl cations
are nonstereospecific.
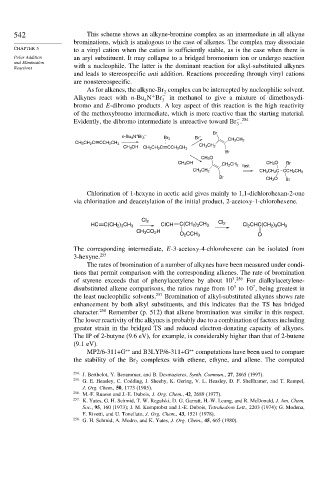
As for alkenes, the alkyne-Br complex can be intercepted by nucleophilic solvent.
2
Alkynes react with n-Bu N Br − in methanol to give a mixture of dimethoxydi-
+
4 3
bromo and E-dibromo products. A key aspect of this reaction is the high reactivity
of the methoxybromo intermediate, which is more reactive than the starting material.
− 254
Evidently, the dibromo intermediate is unreactive toward Br .
3
Br
+ –
n-Bu 4 N Br 3 Br –
Br 2
CH 2 CH 2
CH 3 CH 2 C CCH 2 CH 3
CH 3 OH CH 3 CH 2 C CCH 2 CH 3 CH 3 CH 2
Br
CH 3 O
CH 3 OH CH 3 O Br
CH 2 CH 2 fast
CH 3 CH 2 CH 3 CH 2 C CCH 2 CH 3
Br CH 3 O Br
Chlorination of 1-hexyne in acetic acid gives mainly to 1,1-dichlorohexan-2-one
via chlorination and deacetylation of the initial product, 2-acetoxy-1-chlorohexene.
Cl 2
ClCH Cl 2
HC C(CH 2 ) 3 CH 3 C(CH 2 ) 3 CH 3 Cl 2 CHC(CH 2 ) 3 CH 3
CH 3 CO 2 H
O 2 CCH 3 O
The corresponding intermediate, E-3-acetoxy-4-chlorohexene can be isolated from
3-hexyne. 255
The rates of bromination of a number of alkynes have been measured under condi-
tions that permit comparison with the corresponding alkenes. The rate of bromination
3 256
of styrene exceeds that of phenylacetylene by about 10 . For dialkylacetylene-
7
3
disubstituted alkene comparisons, the ratios range from 10 to 10 , being greatest in
the least nucleophilic solvents. 257 Bromination of alkyl-substituted alkynes shows rate
enhancement by both alkyl substituents, and this indicates that the TS has bridged
character. 258 Remember (p. 512) that alkene bromination was similar in this respect.
The lower reactivity of the alkynes is probably due to a combination of factors including
greater strain in the bridged TS and reduced electron-donating capacity of alkynes.
The IP of 2-butyne (9.6 eV), for example, is considerably higher than that of 2-butene
(9.1 eV).
∗∗
∗∗
MP2/6-311+G and B3LYP/6-311+G computations have been used to compare
the stability of the Br complexes with ethene, ethyne, and allene. The computed
2
254 J. Berthelot, Y. Benammar, and B. Desmazieres, Synth. Commun., 27, 2865 (1997).
255
G. E. Heasley, C. Codding, J. Sheehy, K. Gering, V. L. Heasley, D. F. Shellhamer, and T. Rempel,
J. Org. Chem., 50, 1773 (1985).
256 M.-F. Ruasse and J.-E. Dubois, J. Org. Chem., 42, 2689 (1977).
257 K. Yates, G. H. Schmid, T. W. Regulski, D. G. Garratt, H.-W. Leung, and R. McDonald, J. Am. Chem.
Soc., 95, 160 (1973); J. M. Kornprobst and J.-E. Dubois, Tetrahedron Lett., 2203 (1974); G. Modena,
F. Rivetti, and U. Tonellato, J. Org. Chem., 43, 1521 (1978).
258
G. H. Schmid, A. Modro, and K. Yates, J. Org. Chem., 45, 665 (1980).