Page 563 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 563
544 A salient result of these studies is that ionization is energetically prohibitive, at least in
the gas phase. This finding is consistent with experimental studies that indicate strong
CHAPTER 5 acceleration in polar solvents or in the presence of acids that can facilitate ionization.
Polar Addition
and Elimination
Reactions
5.9.3. Mercuration of Alkynes
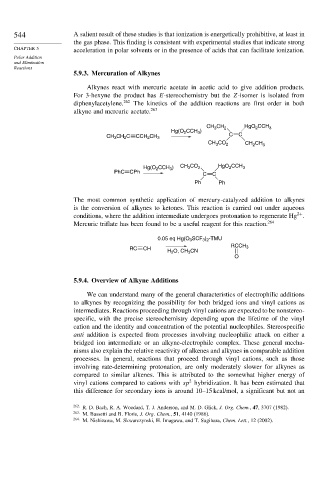
Alkynes react with mercuric acetate in acetic acid to give addition products.
For 3-hexyne the product has E-stereochemistry but the Z-isomer is isolated from
diphenylacetylene. 262 The kinetics of the addition reactions are first order in both
alkyne and mercuric acetate. 263
CH CH 2 HgO CCH 3
2
3
Hg(O 2 CCH 3 )
CH 3 CH 2 C CCH 2 CH 3 C C
CH CO 2 CH CH 3
3
2
Hg(O 2 CCH 3 ) CH CO 2 HgO CCH 3
3
2
PhC CPh
C C
Ph Ph
The most common synthetic application of mercury-catalyzed addition to alkynes
is the conversion of alkynes to ketones. This reaction is carried out under aqueous
2+
conditions, where the addition intermediate undergoes protonation to regenerate Hg .
Mercuric triflate has been found to be a useful reagent for this reaction. 264
0.05 eq Hg(O 3 SCF 3 ) 2 -TMU
RCCH 3
RC CH
H 2 O, CH 3 CN
O
5.9.4. Overview of Alkyne Additions
We can understand many of the general characteristics of electrophilic additions
to alkynes by recognizing the possibility for both bridged ions and vinyl cations as
intermediates. Reactions proceeding through vinyl cations are expected to be nonstereo-
specific, with the precise stereochemistry depending upon the lifetime of the vinyl
cation and the identity and concentration of the potential nucleophiles. Stereospecific
anti addition is expected from processes involving nucleophilic attack on either a
bridged ion intermediate or an alkyne-electrophile complex. These general mecha-
nisms also explain the relative reactivity of alkenes and alkynes in comparable addition
processes. In general, reactions that proceed through vinyl cations, such as those
involving rate-determining protonation, are only moderately slower for alkynes as
compared to similar alkenes. This is attributed to the somewhat higher energy of
2
vinyl cations compared to cations with sp hybridization. It has been estimated that
this difference for secondary ions is around 10–15 kcal/mol, a significant but not an
262 R. D. Bach, R. A. Woodard, T. J. Anderson, and M. D. Glick, J. Org. Chem., 47, 3707 (1982).
263 M. Bassetti and B. Floris, J. Org. Chem., 51, 4140 (1986).
264
M. Nishizawa, M. Skwarczynski, H. Imagawa, and T. Sugihara, Chem. Lett., 12 (2002).