Page 565 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 565
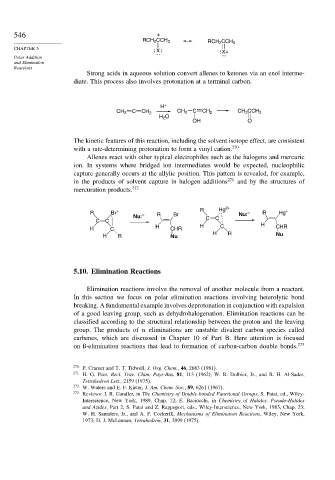
546 +
RCH 2 CCH 3
RCH 2 CCH 3
CHAPTER 5
X X+
Polar Addition
and Elimination
Reactions
Strong acids in aqueous solution convert allenes to ketones via an enol interme-
diate. This process also involves protonation at a terminal carbon.
H +
CH 2 C CH 2 CH 3 C CH 2 CH 3 CCH 3
H 2 O
OH O
The kinetic features of this reaction, including the solvent isotope effect, are consistent
with a rate-determining protonation to form a vinyl cation. 270
Allenes react with other typical electrophiles such as the halogens and mercuric
ion. In systems where bridged ion intermediates would be expected, nucleophilic
capture generally occurs at the allylic position. This pattern is revealed, for example,
in the products of solvent capture in halogen additions 271 and by the structures of
mercuration products. 272
R Br + R Hg 2+ Nu: – R Hg +
Nu: – R Br C C
C C
H C H CHR H C H CHR
H R Nu
H R Nu
5.10. Elimination Reactions
Elimination reactions involve the removal of another molecule from a reactant.
In this section we focus on polar elimination reactions involving heterolytic bond
breaking. A fundamental example involves deprotonation in conjunction with expulsion
of a good leaving group, such as dehydrohalogenation. Elimination reactions can be
classified according to the structural relationship between the proton and the leaving
group. The products of eliminations are unstable divalent carbon species called
carbenes, which are discussed in Chapter 10 of Part B. Here attention is focused
on ß-elimination reactions that lead to formation of carbon-carbon double bonds. 273
270 P. Cramer and T. T. Tidwell, J. Org. Chem., 46, 2683 (1981).
271
H. G. Peer, Recl. Trav. Chim. Pays-Bas, 81, 113 (1962); W. R. Dolbier, Jr., and B. H. Al-Sader,
Tetrahedron Lett., 2159 (1975).
272 W. Waters and E. F. Kieter, J. Am. Chem. Soc., 89, 6261 (1967).
273
Reviews: J. R. Gandler, in The Chemistry of Double-bonded Functional Groups, S. Patai, ed., Wiley-
Interscience, New York, 1989, Chap. 12; E. Baciocchi, in Chemistry of Halides, Pseudo-Halides
and Azides, Part 2, S. Patai and Z. Rappoport, eds., Wiley-Interscience, New York, 1983, Chap. 23;
W. H. Saunders, Jr., and A. F. Cockerill, Mechanisms of Elimination Reactions, Wiley, New York,
1973; D. J. McLennan, Tetrahedron, 31, 2999 (1975).