Page 569 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 569
550 The variable transition state theory allows discussion of reactions proceeding
through TSs of intermediate character in terms of the limiting mechanistic types. These
CHAPTER 5 are called “E1cb-like” and “E1-like,” as illustrated in Figure 5.11. The most important
Polar Addition factors to be considered are: (1) the nature of the leaving group, (2) electronic and
and Elimination
Reactions steric effect of substituents in the reactant molecule, (3) the nature of the base, and (4)
solvent effects.
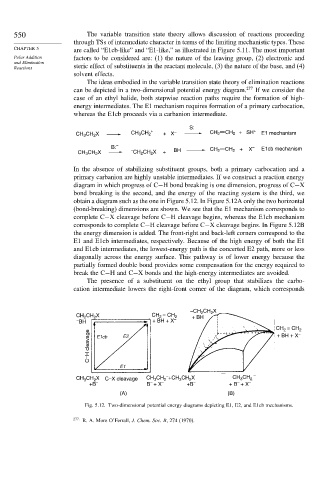
The ideas embodied in the variable transition state theory of elimination reactions
can be depicted in a two-dimensional potential energy diagram. 277 If we consider the
case of an ethyl halide, both stepwise reaction paths require the formation of high-
energy intermediates. The E1 mechanism requires formation of a primary carbocation,
whereas the E1cb proceeds via a carbanion intermediate.
S:
+ +
CH 3 CH 2 X CH 3 CH 2 + X – CH 2 CH 2 + SH E1 mechanism
B: – CH 2 CH 2 + X – E1cb mechanism
CH 3 CH 2 X – CH 2 CH 2 X + BH
In the absence of stabilizing substituent groups, both a primary carbocation and a
primary carbanion are highly unstable intermediates. If we construct a reaction energy
diagram in which progress of C−H bond breaking is one dimension, progress of C−X
bond breaking is the second, and the energy of the reacting system is the third, we
obtain a diagram such as the one in Figure 5.12. In Figure 5.12A only the two horizontal
(bond-breaking) dimensions are shown. We see that the E1 mechanism corresponds to
complete C−X cleavage before C−H cleavage begins, whereas the E1cb mechanism
corresponds to complete C−H cleavage before C−X cleavage begins. In Figure 5.12B
the energy dimension is added. The front-right and back-left corners correspond to the
E1 and E1cb intermediates, respectively. Because of the high energy of both the E1
and E1cb intermediates, the lowest-energy path is the concerted E2 path, more or less
diagonally across the energy surface. This pathway is of lower energy because the
partially formed double bond provides some compensation for the energy required to
break the C−H and C−X bonds and the high-energy intermediates are avoided.
The presence of a substituent on the ethyl group that stabilizes the carbo-
cation intermediate lowers the right-front corner of the diagram, which corresponds
CH X
–CH 2 2
CH CH X CH = CH 2 + BH
2
2
2
– BH + BH + X –
CH = CH – 2
2
C–H cleavage
+ BH + X
E2
E1cb
E1
–
CH CH X C–X cleavage CH CH +CH CH X CH CH 2 –
3
3
2
3
2
3
2
–
–
+B – B + X – +B – + B + X –
(A) (B)
Fig. 5.12. Two-dimensional potential energy diagrams depicting E1, E2, and E1cb mechanisms.
277
R. A. More O’Ferrall, J. Chem. Soc. B, 274 (1970).