Page 574 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 574
In the E1 mechanism, the leaving group is completely ionized before C−H bond 555
breaking occurs. The direction of the elimination therefore depends on the structure
of the carbocation and the identity of the base involved in the proton transfer that SECTION 5.10
follows C−X heterolysis. Because of the high energy of the carbocation intermediate, Elimination Reactions
quite weak bases can effect proton removal. The solvent can serve this function. The
counterion formed in the ionization step can also act as the proton acceptor.
+ + +
B: H CH 2 CHCH CH CH 3 CH CHCH CH CH 3 CH CH CHCH CH 3
3
2
2
2
2
3
2
H
:B
CH 2 CHCH CH CH 3 CH CH CHCH CH 3
2
3
2
2
The product composition of the alkenes formed in E1 elimination reaction usually
favors the more-substituted alkene, and therefore the more stable one. This indicates
that the energies of the product-determining TSs parallel those of the isomeric alkenes.
However, since the activation energy for proton removal from a carbocation is low, the
TS should resemble the carbocation intermediate much more than the alkene product
(Hammond postulate; Section 3.3.2.2). In the carbocation there is hyperconjugation
involving each ß-hydrogen. 281 Since the hyperconjugation structures possess some
double-bondcharacter,theinteractionwithhydrogenisgreatestatmorehighlysubstituted
carbons; that is, there will be greater weakening of C−H bonds and more double-bond
characteratmorehighlysubstitutedcarbonatoms.Thisstructuraleffectinthecarbocation
intermediate governs the direction of elimination and leads to the preferential formation
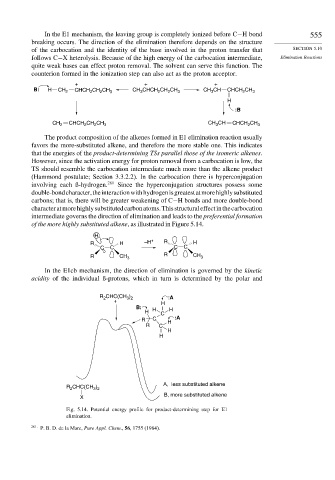
of the more highly substituted alkene, as illustrated in Figure 5.14.
H
R H –H + R H
C C C C
R CH 3 R CH 3
In the E1cb mechanism, the direction of elimination is governed by the kinetic
acidity of the individual ß-protons, which in turn is determined by the polar and
R CHC(CH ) :A
3 2
2
+ H
B: H H
H C
R C H :A
R C
H
H
A, less substituted alkene
R CHC(CH )
2
3 2
B, more substituted alkene
X
Fig. 5.14. Potential energy profile for product-determining step for E1
elimination.
281 P. B. D. de la Mare, Pure Appl. Chem., 56, 1755 (1984).