Page 577 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 577
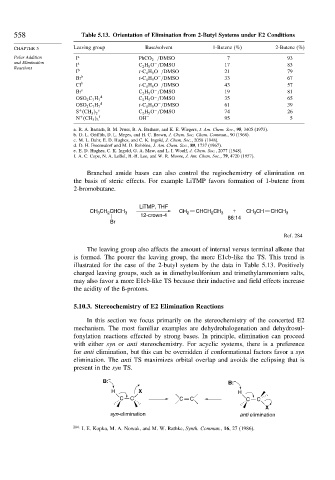
558 Table 5.13. Orientation of Elimination from 2-Butyl Systems under E2 Conditions
CHAPTER 5 Leaving group Base/solvent 1-Butene (%) 2-Butene (%)
Polar Addition I a PhCO 2 /DMSO 7 93
−
and Elimination I a C 2 H 5 O /DMSO 17 83
−
Reactions
I b t-C 4 H 9 O /DMSO 21 79
−
Br b t-C 4 H 9 O /DMSO 33 67
−
Cl b t-C 4 H 9 O /DMSO 43 57
−
−
Br c C 2 H 5 O /DMSO 19 81
d −
OSO 2 C 7 H 7 C 2 H 5 O /DMSO 35 65
d −
OSO 2 C 7 H 7 t-C 4 H 9 O /DMSO 61 39
−
+ e C 2 H 5 O /DMSO 74 26
S CH 3 2
+ f OH − 95 5
N CH 3 3
a. R. A. Bartsch, B. M. Pruss, B. A. Bushaw, and K. E. Wiegers, J. Am. Chem. Soc., 95, 3405 (1973).
b. D. L. Griffith, D. L. Meges, and H. C. Brown, J. Chem. Soc. Chem. Commun., 90 (1968).
c. M. L. Dahr, E. D. Hughes, and C. K. Ingold, J. Chem. Soc., 2058 (1948).
d. D. H. Froemsdorf and M. D. Robbins, J. Am. Chem. Soc., 89, 1737 (1967).
e. E. D. Hughes, C. K. Ingold, G. A. Maw, and L. I. Woolf, J. Chem. Soc., 2077 (1948).
f. A. C. Cope, N. A. LeBel, H.-H. Lee, and W. R. Moore, J. Am. Chem. Soc., 79, 4720 (1957).
Branched amide bases can also control the regiochemistry of elimination on
the basis of steric effects. For example LiTMP favors formation of 1-butene from
2-bromobutane.
LiTMP, THF
CH CHCH CH CHCH CH + CH CH CHCH
CH 3 2 3 2 2 3 3 3
12-crown-4 86:14
Br
Ref. 284
The leaving group also affects the amount of internal versus terminal alkene that
is formed. The poorer the leaving group, the more E1cb-like the TS. This trend is
illustrated for the case of the 2-butyl system by the data in Table 5.13. Positively
charged leaving groups, such as in dimethylsulfonium and trimethylammonium salts,
may also favor a more E1cb-like TS because their inductive and field effects increase
the acidity of the ß-protons.
5.10.3. Stereochemistry of E2 Elimination Reactions
In this section we focus primarily on the stereochemistry of the concerted E2
mechanism. The most familiar examples are dehydrohalogenation and dehydrosul-
fonylation reactions effected by strong bases. In principle, elimination can proceed
with either syn or anti stereochemistry. For acyclic systems, there is a preference
for anti elimination, but this can be overridden if conformational factors favor a syn
elimination. The anti TS maximizes orbital overlap and avoids the eclipsing that is
present in the syn TS.
B: – B: –
H X H
C C C C C C
X
syn-elimination anti elimination
284
I. E. Kopka, M. A. Nowak, and M. W. Rathke, Synth. Commun., 16, 27 (1986).