Page 576 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 576
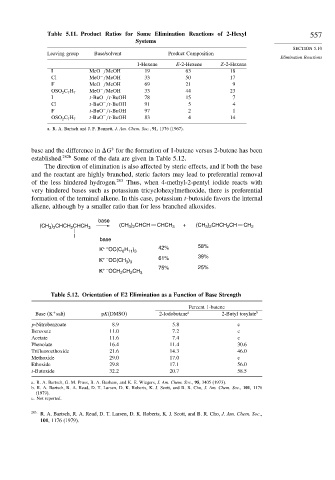
Table 5.11. Product Ratios for Some Elimination Reactions of 2-Hexyl 557
Systems
SECTION 5.10
Leaving group Base/solvent Product Composition
Elimination Reactions
1-Hexene E-2-Hexene Z-2-Hexene
−
I MeO /MeOH 19 63 18
Cl MeO /MeOH 33 50 17
−
F MeO /MeOH 69 21 9
−
MeO /MeOH 33 44 23
−
OSO 2 C 7 H 7
−
I t-BuO /t-BuOH 78 15 7
−
Cl t-BuO /t-BuOH 91 5 4
−
F t-BuO /t-BuOH 97 2 1
t-BuO /t-BuOH 83 4 14
−
OSO 2 C 7 H 7
a. R. A. Bartsch and J. F. Bunnett, J. Am. Chem. Soc., 91, 1376 (1967).
‡
base and the difference in G for the formation of 1-butene versus 2-butene has been
established. 282b Some of the data are given in Table 5.12.
The direction of elimination is also affected by steric effects, and if both the base
and the reactant are highly branched, steric factors may lead to preferential removal
of the less hindered hydrogen. 283 Thus, when 4-methyl-2-pentyl iodide reacts with
very hindered bases such as potassium tricyclohexylmethoxide, there is preferential
formation of the terminal alkene. In this case, potassium t-butoxide favors the internal
alkene, although by a smaller ratio than for less branched alkoxides.
base
(CH ) CHCH CHCH 3 (CH ) CHCH CHCH 3 + (CH ) CHCH CH CH 2
3 2
3 2
2
2
3 2
I
base
K + – OC(C H ) 42% 58%
6 11 3
K + – OC(CH ) 61% 39%
3 3
75% 25%
K + – OCH CH CH 3
2
2
Table 5.12. Orientation of E2 Elimination as a Function of Base Strength
Percent 1-butene
Base (K salt) pK(DMSO) 2-Iodobutane a 2-Butyl tosylate b
+
p-Nitrobenzoate 8 9 5 8 c
Benzoate 11 0 7 2 c
Acetate 11 6 7 4 c
Phenolate 16 4 11 4 30.6
Trifluoroethoxide 21 6 14 3 46.0
Methoxide 29 0 17 0 c
Ethoxide 29 8 17 1 56.0
t-Butoxide 32 2 20 7 58.5
a. R. A. Bartsch, G. M. Pruss, B. A. Bushaw, and K. E. Wiegers, J. Am. Chem. Soc., 95, 3405 (1973).
b. R. A. Bartsch, R. A. Read, D. T. Larsen, D. K. Roberts, K. J. Scott, and B. R. Cho, J. Am. Chem. Soc., 101, 1176
(1979).
c. Not reported.
283
R. A. Bartsch, R. A. Read, D. T. Larsen, D. K. Roberts, K. J. Scott, and B. R. Cho, J. Am. Chem. Soc.,
101, 1176 (1979).