Page 581 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 581
562 Cl
1.437Å
CHAPTER 5 2.176Å
Polar Addition
and Elimination
Reactions 1.433Å
1.141Å
1.384Å
2.100Å
1.195Å
1.417Å
Cl
F
1.430Å
Cl
1.417Å
2.195Å
1.492Å
1.104Å
2.109Å F
1.380Å
1.199Å Cl
F
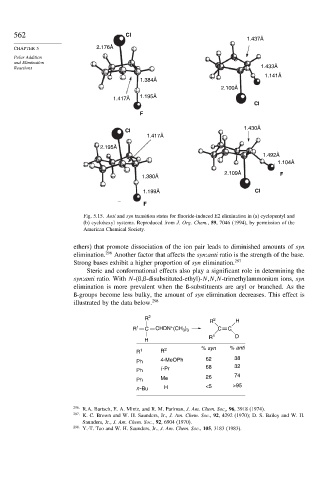
Fig. 5.15. Anti and syn transition states for fluoride-induced E2 elimination in (a) cyclopentyl and
(b) cyclohexyl systems. Reproduced from J. Org. Chem., 59, 7046 (1994), by permission of the
American Chemical Society.
ethers) that promote dissociation of the ion pair leads to diminished amounts of syn
elimination. 296 Another factor that affects the syn:anti ratio is the strength of the base.
Strong bases exhibit a higher proportion of syn elimination. 297
Steric and conformational effects also play a significant role in determining the
syn:anti ratio. With N-(ß,ß-disubstituted-ethyl)-N,N,N-trimethylammonium ions, syn
elimination is more prevalent when the ß-substituents are aryl or branched. As the
ß-groups become less bulky, the amount of syn elimination decreases. This effect is
illustrated by the data below. 298
R 2 2
R H
+
R 1 C CHDN (CH ) C C
3 3
R 1 D
H
R 1 R 2 % syn % anti
Ph 4-MeOPh 62 38
Ph i -Pr 68 32
Ph Me 26 74
n -Bu H <5 >95
296
R.A. Bartsch, E. A. Mintz, and R. M. Parlman, J. Am. Chem. Soc., 96, 3918 (1974).
297 K. C. Brown and W. H. Saunders, Jr., J. Am. Chem. Soc., 92, 4292 (1970); D. S. Bailey and W. H.
Saunders, Jr., J. Am. Chem. Soc., 92, 6904 (1970).
298
Y.-T. Tao and W. H. Saunders, Jr., J. Am. Chem. Soc., 105, 3183 (1983).