Page 583 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 583
564 This elimination reaction is the reverse of acid-catalyzed hydration, which was
discussed in Section 5.2. Since a carbocation or closely related species is the inter-
CHAPTER 5 mediate, the elimination step is expected to favor the more-substituted alkene. The
Polar Addition E1 mechanism also explains the trends in relative reactivity. Tertiary alcohols are the
and Elimination
Reactions most reactive, and reactivity decreases going to secondary and primary alcohols. Also
in accord with the E1 mechanism is the fact that rearranged products are found in
cases where a carbocation intermediate would be expected to rearrange.
R
CCHR' R CCHR' R
R 3 3
+ H + R CCHR' R CCHR' R 2 C C
3
2
+
OH O H 2 + + R'
For some alcohols, exchange of the hydroxyl group with solvent competes with
dehydration. 301 This exchange indicates that the carbocation can undergo S 1 capture
N
in competition with elimination. Under conditions where proton removal is rate deter-
mining, it would be expected that a significant isotope effect would be seen, which is,
in fact, observed.
H *
H SO 4
2
PhCHCHPh PhCH CHPh
H O
2
OH k /k D = 1.8
H
Ref. 302
5.10.5. Eliminations Reactions Not Involving C−H Bonds
The discussion of elimination processes thus far has focused on reactions that
involve removal of a proton bound to a ß-carbon, but it is the electrons in the C−H
bond that are essential to the elimination process. Compounds bearing other substituents
that can release electrons undergo -eliminations. Many such reactions are known,
and they are frequently stereospecific.
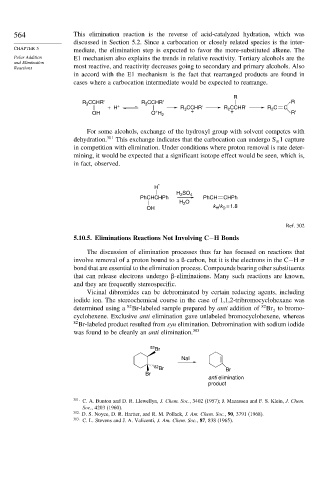
Vicinal dibromides can be debrominated by certain reducing agents, including
iodide ion. The stereochemical course in the case of 1,1,2-tribromocyclohexane was
determined using a 82 Br-labeled sample prepared by anti addition of 82 Br to bromo-
2
cyclohexene. Exclusive anti elimination gave unlabeled bromocyclohexene, whereas
82
Br-labeled product resulted from syn elimination. Debromination with sodium iodide
was found to be cleanly an anti elimination. 303
82
Br
NaI
82 Br Br
Br
anti elimination
product
301
C. A. Bunton and D. R. Llewellyn, J. Chem. Soc., 3402 (1957); J. Manassen and F. S. Klein, J. Chem.
Soc., 4203 (1960).
302 D. S. Noyce, D. R. Hartter, and R. M. Pollack, J. Am. Chem. Soc., 90, 3791 (1968).
303
C. L. Stevens and J. A. Valicenti, J. Am. Chem. Soc., 87, 838 (1965).