Page 584 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 584
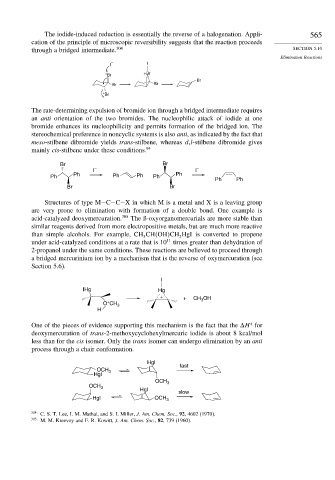
The iodide-induced reduction is essentially the reverse of a halogenation. Appli- 565
cation of the principle of microscopic reversibility suggests that the reaction proceeds
through a bridged intermediate. 304 SECTION 5.10
Elimination Reactions
– I
I
+Br
*Br
Br
Br Br
*Br
The rate-determining expulsion of bromide ion through a bridged intermediate requires
an anti orientation of the two bromides. The nucleophilic attack of iodide at one
bromide enhances its nucleophilicity and permits formation of the bridged ion. The
stereochemical preference in noncyclic systems is also anti, as indicated by the fact that
meso-stilbene dibromide yields trans-stilbene, whereas d,l-stilbene dibromide gives
mainly cis-stilbene under these conditions. 94
Br Br
I – I –
Ph Ph
Ph Ph Ph Ph
Ph Ph
Br Br
Structures of type M−C−C−X in which M is a metal and X is a leaving group
are very prone to elimination with formation of a double bond. One example is
acid-catalyzed deoxymercuration. 305 The ß-oxyorganomercurials are more stable than
similar reagents derived from more electropositive metals, but are much more reactive
than simple alcohols. For example, CH CH OH CH HgI is converted to propene
3
2
11
under acid-catalyzed conditions at a rate that is 10 times greater than dehydration of
2-propanol under the same conditions. These reactions are believed to proceed through
a bridged mercurinium ion by a mechanism that is the reverse of oxymercuration (see
Section 5.6).
I
IHg Hg
+ + CH OH
+
O CH 3 3
H
‡
One of the pieces of evidence supporting this mechanism is the fact that the H for
deoxymercuration of trans-2-methoxycyclohexylmercuric iodide is about 8 kcal/mol
less than for the cis isomer. Only the trans isomer can undergo elimination by an anti
process through a chair conformation.
HgI
fast
OCH 3
HgI
OCH 3
OCH 3
HgI
slow
HgI OCH 3
304 C. S. T. Lee, I. M. Mathai, and S. I. Miller, J. Am. Chem. Soc., 92, 4602 (1970).
305
M. M. Kreevoy and F. R. Kowitt, J. Am. Chem. Soc., 82, 739 (1960).