Page 588 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 588
General References 569
PROBLEMS
G. V. Boyd, in The Chemistry of Triple-Bonded Functional Groups, Supplement 2, S. Patai, ed., John Wiley
& Sons, New York, 1994, Chap. 6.
A. F. Cockerill and R. G. Harrison, The Chemistry of Double-Bonded Functional Groups, Part 1, S. Patai,
ed., John Wiley & Sons, New York, 1977, Chap. 4.
P. B. de la Mare and R. Bolton, Electrophilic Additions to Unsaturated Systems, 2nd Edition, Elsevier,
New York, 1982.
J. G. Gandler, in The Chemistry of Double-Bonded Functional Groups, Supplement A, Vol. 2, S. Patai, ed.,
John Wiley & Sons, New York, 1989, Chap. 12.
G. H. Schmid, in The Chemistry of Double-Bonded Functional Groups, Supplement A, Vol. 2, S. Patai, ed.,
John Wiley & Sons, New York, 1989, Chap. 11.
P. J. Stang and F. Diederich, eds., Modern Acetylene Chemistry, VCH Publishers, Weinheim, 1995.
W. H. Saunders, Jr., and A. F. Cockerill, Mechanisms of Elimination Reactions, John Wiley & Sons,
New York, 1973.
Problems
(References for these problems will be found on page 1160.)
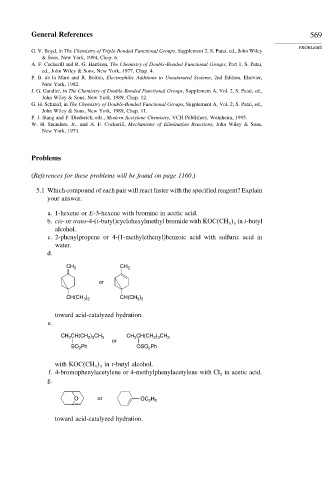
5.1 Which compound of each pair will react faster with the specified reagent? Explain
your answer.
a. 1-hexene or E-3-hexene with bromine in acetic acid.
b. cis-or trans-4-(t-butyl)cyclohexylmethyl bromide with KOC CH in t-butyl
3 3
alcohol.
c. 2-phenylpropene or 4-(1-methylethenyl)benzoic acid with sulfuric acid in
water.
d.
CH 2 CH 3
or
CH(CH ) CH(CH )
3 2
3 2
toward acid-catalyzed hydration.
e.
CH CH(CH ) CH 3 CH 3 CH(CH ) CH 3
2 3
2 3
3
or
SO Ph OSO Ph
2
2
with KOC CH in t-butyl alcohol.
3 3
f. 4-bromophenylacetylene or 4-methylphenylacetylene with Cl in acetic acid.
2
g.
O or OC H
2 5
toward acid-catalyzed hydration.