Page 592 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 592
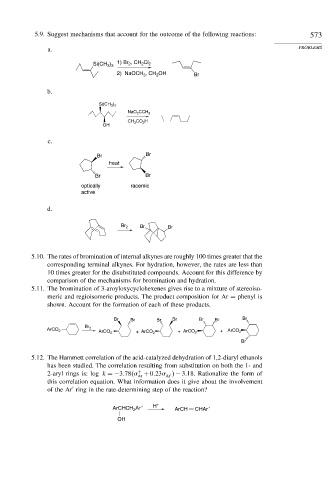
5.9. Suggest mechanisms that account for the outcome of the following reactions: 573
a. PROBLEMS
1) Br , CH Cl
Si(CH 3 ) 3 2 2 2
2) NaOCH , CH OH Br
3
3
b.
Si(CH 3 ) 3
NaO 2 CCH 3
CH 3 CO 2 H
OH
c.
Br Br
heat
Br Br
optically racemic
active
d.
Br 2 Br Br
5.10. The rates of bromination of internal alkynes are roughly 100 times greater that the
corresponding terminal alkynes. For hydration, however, the rates are less than
10 times greater for the disubstituted compounds. Account for this difference by
comparison of the mechanisms for bromination and hydration.
5.11. The bromination of 3-aroyloxycyclohexenes gives rise to a mixture of stereoiso-
meric and regioisomeric products. The product composition for Ar = phenyl is
shown. Account for the formation of each of these products.
Br Br Br Br Br Br Br
Br 2
ArCO 2
ArCO 2 + ArCO 2 + ArCO 2 + ArCO 2
Br
5.12. The Hammett correlation of the acid-catalyzed dehydration of 1,2-diaryl ethanols
has been studied. The correlation resulting from substitution on both the 1- and
+
2-aryl rings is: log k =−3 78
+ 0 23
− 3 18. Rationalize the form of
Ar Ar
this correlation equation. What information does it give about the involvement
of the Ar ring in the rate-determining step of the reaction?
ArCHCH Ar ′ H + ArCH CHAr ′
2
OH