Page 595 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 595
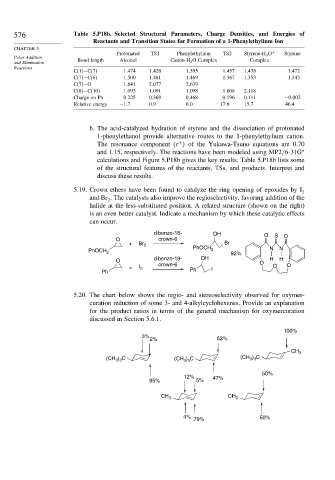
576 Table 5.P18b. Selected Structural Parameters, Charge Densities, and Energies of
Reactants and Transition States for Formation of a 1-Phenylethylium Ion
CHAPTER 5
Protonated TS1 Phenylethylium TS2 Styrene-H 3 O + Styrene
Polar Addition
and Elimination Bond length Alcohol Cation-H 2 O Complex Complex
Reactions
C(1)−C(7) 1 474 1 420 1 395 1 457 1.478 1.472
C(7)−C(8) 1 500 1 481 1 469 1 367 1.353 1.343
C(7)−O 1 641 2 077 2 639
C(8)−C(10) 1 093 1 091 1 098 1 608 2.118
Charge on Ph 0 225 0 369 0 468 0 196 0.111 −0 002
Relative energy −1 7 0 9 0 0 17 6 15.7 46.4
b. The acid-catalyzed hydration of styrene and the dissociation of protonated
1-phenylethanol provide alternative routes to the 1-phenylethylium cation.
+
The resonance component (r of the Yukawa-Tsuno equations are 0.70
and 1.15, respectively. The reactions have been modeled using MP2/6-31G ∗
calculations and Figure 5.P18b gives the key results. Table 5.P18b lists some
of the structural features of the reactants, TSs, and products. Interpret and
discuss these results.
5.19. Crown ethers have been found to catalyze the ring opening of epoxides by I 2
and Br . The catalysts also improve the regioselectivity, favoring addition of the
2
halide at the less-substituted position. A related structure (shown on the right)
is an even better catalyst. Indicate a mechanism by which these catalytic effects
can occur.
dibenzo-18- OH O S
O crown-6 O
+ Br 2 Br
PhOCH 2 PhOCH 2 92% N N
dibenzo-18- OH H H
O crown-6 O
+ I 2 I O O
Ph Ph
5.20. The chart below shows the regio- and stereoselectivity observed for oxymer-
curation reduction of some 3- and 4-alkylcyclohexenes. Provide an explanation
for the product ratios in terms of the general mechanism for oxymercuration
discussed in Section 5.6.1.
100%
3%
2% 53%
CH 3
(CH ) C (CH 3 3 (CH ) C
) C
3 3
3 3
50%
12% 47%
95% 5%
CH 3 CH 3
4% 50%
79%