Page 591 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 591
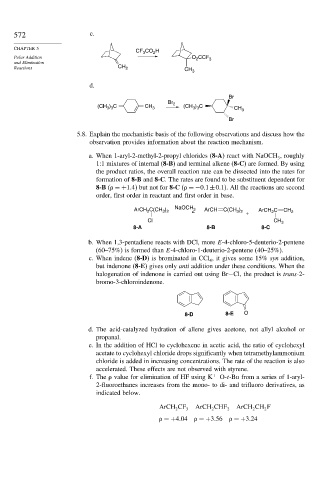
572 c.
CHAPTER 5
CF CO H
3
2
Polar Addition O CCF 3
2
and Elimination
Reactions CH 2 CH 3
d.
Br
Br 2
(CH 3 ) 3 C CH 3 (CH 3 ) 3 C CH 3
Br
5.8. Explain the mechanistic basis of the following observations and discuss how the
observation provides information about the reaction mechanism.
a. When 1-aryl-2-methyl-2-propyl chlorides (8-A) react with NaOCH , roughly
3
1:1 mixtures of internal (8-B) and terminal alkene (8-C) are formed. By using
the product ratios, the overall reaction rate can be dissected into the rates for
formation of 8-B and 8-C. The rates are found to be substituent dependent for
8-B (
=+1 4) but not for 8-C (
=−0 1±0 1). All the reactions are second
order, first order in reactant and first order in base.
ArCH C(CH ) NaOCH 3 ArCH C(CH ) ArCH C CH
3 2
3 2
2
+ 2 2
Cl CH 3
8-A 8-B 8-C
b. When 1,3-pentadiene reacts with DCl, more E-4-chloro-5-deuterio-2-pentene
(60–75%) is formed than E-4-chloro-1-deuterio-2-pentene (40–25%).
c. When indene (8-D) is brominated in CCl , it gives some 15% syn addition,
4
but indenone (8-E) gives only anti addition under these conditions. When the
halogenation of indenone is carried out using Br−Cl, the product is trans-2-
bromo-3-chloroindenone.
8-D 8-E O
d. The acid-catalyzed hydration of allene gives acetone, not allyl alcohol or
propanal.
e. In the addition of HCl to cyclohexene in acetic acid, the ratio of cyclohexyl
acetate to cyclohexyl chloride drops significantly when tetramethylammonium
chloride is added in increasing concentrations. The rate of the reaction is also
accelerated. These effects are not observed with styrene.
f. The
value for elimination of HF using K +− O-t-Bu from a series of 1-aryl-
2-fluoroethanes increases from the mono- to di- and trifluoro derivatives, as
indicated below.
ArCH CF ArCH CHF ArCH CH F
2 3 2 2 2 2
=+4 04
=+3 56
=+3 24