Page 590 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 590
h. 571
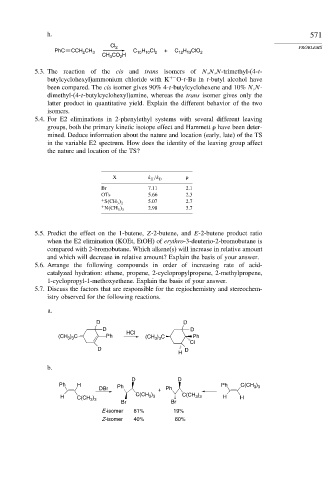
Cl 2 PROBLEMS
H Cl
PhC CCH CH 3 C 10 10 2 + C H ClO 2
12 13
2
CH CO H
3
2
5.3. The reaction of the cis and trans isomers of N,N,N-trimethyl-(4-t-
butylcyclohexyl)ammonium chloride with K +− O-t-Bu in t-butyl alcohol have
been compared. The cis isomer gives 90% 4-t-butylcyclohexene and 10% N,N-
dimethyl-(4-t-butylcyclohexyl)amine, whereas the trans isomer gives only the
latter product in quantitative yield. Explain the different behavior of the two
isomers.
5.4. For E2 eliminations in 2-phenylethyl systems with several different leaving
groups, both the primary kinetic isotope effect and Hammett
have been deter-
mined. Deduce information about the nature and location (early, late) of the TS
in the variable E2 spectrum. How does the identity of the leaving group affect
the nature and location of the TS?
X k H /k D
Br 7.11 2.1
OTs 5.66 2.3
+ 5.07 2.7
S CH 3 2
+ 2.98 3.7
N CH 3 3
5.5. Predict the effect on the 1-butene, Z-2-butene, and E-2-butene product ratio
when the E2 elimination (KOEt, EtOH) of erythro-3-deuterio-2-bromobutane is
compared with 2-bromobutane. Which alkene(s) will increase in relative amount
and which will decrease in relative amount? Explain the basis of your answer.
5.6. Arrange the following compounds in order of increasing rate of acid-
catalyzed hydration: ethene, propene, 2-cyclopropylpropene, 2-methylpropene,
1-cyclopropyl-1-methoxyethene. Explain the basis of your answer.
5.7. Discuss the factors that are responsible for the regiochemistry and stereochem-
istry observed for the following reactions.
a.
D D
D D
HCl
(CH ) C Ph (CH 3 3 Ph
) C
3 3
Cl
D D
H
b.
D D
Ph H Ph Ph C(CH 3 ) 3
DBr + Ph
H C(CH 3 ) 3 C(CH 3 ) 3 C(CH 3 ) 3 H H
Br Br
E-isomer 81% 19%
Z-isomer 40% 60%