Page 570 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 570
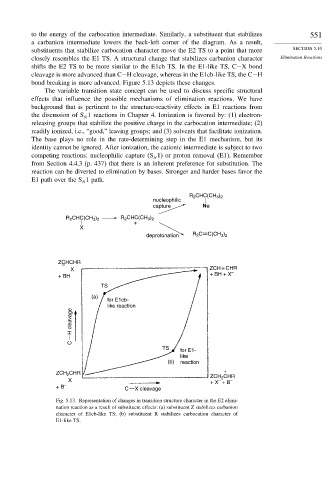
to the energy of the carbocation intermediate. Similarly, a substituent that stabilizes 551
a carbanion intermediate lowers the back-left corner of the diagram. As a result,
substituents that stabilize carbocation character move the E2 TS to a point that more SECTION 5.10
closely resembles the E1 TS. A structural change that stabilizes carbanion character Elimination Reactions
shifts the E2 TS to be more similar to the E1cb TS. In the E1-like TS, C−X bond
cleavage is more advanced than C−H cleavage, whereas in the E1cb-like TS, the C−H
bond breaking is more advanced. Figure 5.13 depicts these changes.
The variable transition state concept can be used to discuss specific structural
effects that influence the possible mechanisms of elimination reactions. We have
background that is pertinent to the structure-reactivity effects in E1 reactions from
the discussion of S 1 reactions in Chapter 4. Ionization is favored by: (1) electron-
N
releasing groups that stabilize the positive charge in the carbocation intermediate; (2)
readily ionized, i.e., “good,” leaving groups; and (3) solvents that facilitate ionization.
The base plays no role in the rate-determining step in the E1 mechanism, but its
identity cannot be ignored. After ionization, the cationic intermediate is subject to two
competing reactions: nucleophilic capture (S 1) or proton removal (E1). Remember
N
from Section 4.4.3 (p. 437) that there is an inherent preference for substitution. The
reaction can be diverted to elimination by bases. Stronger and harder bases favor the
E1 path over the S 1 path.
N
R 2 CHC(CH 3 ) 2
nucleophilic
capture Nu
R 2 CHC(CH 3 ) 2 R 2 CHC(CH 3 ) 2
+
X
deprotonation R 2 C C(CH )
3 2
ZCHCHR
–
X ZCH = CHR
+ BH + BH + X –
TS
(a)
for E1cb-
like reaction
H cleavage
C
TS for E1-
like
(b) reaction
CHR +
ZCH 2
ZCH CHR
2
X + X + B –
–
+ B – C X cleavage
Fig. 5.13. Representation of changes in transition structure character in the E2 elimi-
nation reaction as a result of substituent effects: (a) substituent Z stabilizes carbanion
character of E1cb-like TS; (b) substituent R stabilizes carbocation character of
E1-like TS.