Page 566 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 566
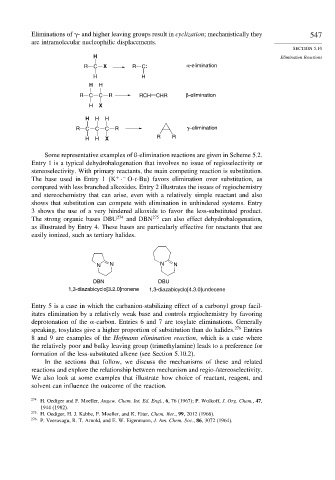
Eliminations of - and higher leaving groups result in cyclization; mechanistically they 547
are intramolecular nucleophilic displacements.
SECTION 5.10
H Elimination Reactions
R C X R C α-elimination
H H
H H
R C C R RCH CHR β-elimination
H X
H H H
R C C C R γ−elimination
H H X R R
Some representative examples of ß-elimination reactions are given in Scheme 5.2.
Entry 1 is a typical dehydrohalogenation that involves no issue of regioselectivity or
stereoselectivity. With primary reactants, the main competing reaction is substitution.
The base used in Entry 1 (K · O-t-Bu) favors elimination over substitution, as
+ −
compared with less branched alkoxides. Entry 2 illustrates the issues of regiochemistry
and stereochemistry that can arise, even with a relatively simple reactant and also
shows that substitution can compete with elimination in unhindered systems. Entry
3 shows the use of a very hindered alkoxide to favor the less-substituted product.
The strong organic bases DBU 274 and DBN 275 can also effect dehydrohalogenation,
as illustrated by Entry 4. These bases are particularly effective for reactants that are
easily ionized, such as tertiary halides.
N N N N
DBN DBU
1,3-diazabicyclo[3.2.0]nonene 1,3-diazabicyclo[4.3.0]undecene
Entry 5 is a case in which the carbanion-stabilizing effect of a carbonyl group facil-
itates elimination by a relatively weak base and controls regiochemistry by favoring
deprotonation of the -carbon. Entries 6 and 7 are tosylate eliminations. Generally
speaking, tosylates give a higher proportion of substitution than do halides. 276 Entries
8 and 9 are examples of the Hofmann elimination reaction, which is a case where
the relatively poor and bulky leaving group (trimethylamine) leads to a preference for
formation of the less-substituted alkene (see Section 5.10.2).
In the sections that follow, we discuss the mechanisms of these and related
reactions and explore the relationship between mechanism and regio-/stereoselectivity.
We also look at some examples that illustrate how choice of reactant, reagent, and
solvent can influence the outcome of the reaction.
274 H. Oediger and F. Moeller, Angew. Chem. Int. Ed. Engl., 6, 76 (1967); P. Wolkoff, J. Org. Chem., 47,
1944 (1982).
275 H. Oediger, H. J. Kabbe, F. Moeller, and K. Eiter, Chem. Ber., 99, 2012 (1966).
276
P. Veeravagu, R. T. Arnold, and E. W. Eigenmann, J. Am. Chem. Soc., 86, 3072 (1964).