Page 786 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 786
769
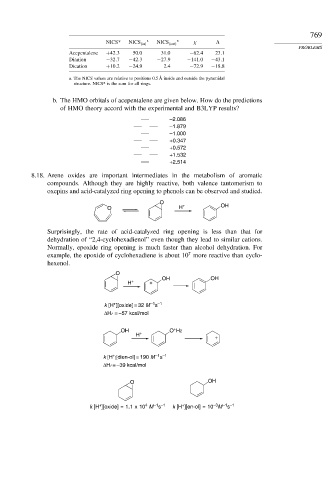
NICS* NICS in a NICS out a
PROBLEMS
Acepentalene +42 3 50.0 31.0 −62 4 23 1
Dianion −32 7 −42 3 −27 9 −141 0 −43 1
Dication +10 2 −24 9 2.4 −72 9 −18 8
a. The NICS values are relative to positions 0.5 Å inside and outside the pyramidal
structure. NICS* is the sum for all rings.
b. The HMO orbitals of acepentalene are given below. How do the predictions
of HMO theory accord with the experimental and B3LYP results?
–2.086
–1.879
–1.000
+0.347
+0.572
+1.532
+2.514
8.18. Arene oxides are important intermediates in the metabolism of aromatic
compounds. Although they are highly reactive, both valence tautomerism to
oxepins and acid-catalyzed ring opening to phenols can be observed and studied.
O
O H + OH
Surprisingly, the rate of acid-catalyzed ring opening is less than that for
dehydration of “2,4-cyclohexadienol” even though they lead to similar cations.
Normally, epoxide ring opening is much faster than alcohol dehydration. For
7
example, the epoxide of cyclohexadiene is about 10 more reactive than cyclo-
hexenol.
O
OH OH
H + +
–1 –1
+
k [H ][oxide] = 32 M s
ΔHr = –57 kcal/mol
+
OH O H2
H +
+
+
–1 –1
k [H ][dien-ol] = 190 M s
ΔHr = –39 kcal/mol
O OH
–1 –1
+
–3
4
+
–1 –1
k [H ][oxide] = 1.1 x 10 M s k [H ][en-ol] = 10 M s