Page 790 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 790
Scheme 9.1. (continued) 773
C. Electrophiles capable of substitution only strongly activated aromatic rings SECTION 9.1
+
+
19 l HC N H HC N + HX HC N H + X – Electrophilic Aromatic
Substitution Reactions
+
20 m N O + HONO + H + N O + H O
2
21 n ArN + N ArNH + HONO + H + ArN + N + 2H O
2
2
a. G. A. Olah and S. J. Kuhn, in Friedel-Crafts and Related Reactions, Vol. III, G. A. Olah, ed., Interscience, New York,
1964, Chapter XLIII.
b. H. P. Braendlin and E. T. McBee, in Friedel-Crafts and Related Reactions, Vol. III, G. A. Olah, ed., Interscience,
New York, 1964, Chapter XLVI.
c. K. L. Nelson, in Friedel-Crafts and Related Reactions, Vol. III, G. A. Olah, ed., Interscience, New York, 1964, Chapter,
XLVII.
d. F. R. Jensen and G. Goldman, in Friedel-Crafts and Related Reactions, Vol. III, G. A. Olah, ed., Interscience, New
York, 1964, Chapter XL.
e. F. A. Drahowzal, in Friedel-Crafts and Related Reactions, Vol. II, G. A. Olah, ed., Interscience, New York, 1964,
Chapter XVII.
f. A. Schreisheim, in Friedel-Crafts and Related Reactions, Vol. II, G. A. Olah, ed., Interscience, New York, 1964,
Chapter XVIII.
g. S. H. Patinkin and B. S. Friedman, in Friedel-Crafts and Related Reactions, Vol. II, G. A. Olah, ed., Interscience,
New York, 1964, Chapter XIV.
h. P. H. Gore, in Friedel-Crafts and Related Reactions, Vol III, G. A. Olah, ed., Interscience, New York, 1964,
Chapter XXXI.
i. Y. Sato, M. Yato, T. Ohwada, S. Saito, and K. Shudo, J. Am. Chem. Soc., 117, 3037 (1995).
j. R. O. C. Norman and R. Taylor, Electrophilic Substitution in Benzenoid Compounds, Elsevier, New York, 1965, Chapter 8.
k. J. E. Hofmann and A. Schreisheim, in Friedel-Crafts and Related Reactions, Vol. II, G. A. Olah, ed., Interscience,
New York, 1964, Chapter XIX.
l. W. Ruske, in Friedel-Crafts and Related Reactions, Vol. III, G. A. Olah, ed., Interscience, New York, 1964,
Chapter XXXII.
m. B. C. Challis, R. J. Higgins, and A. J. Lawson, J. Chem. Soc., Perkin Trans., 2, 1831 (1972).
n. H. Zollinger, Azo and Diazo Chemistry, transl. H. E. Nursten, Interscience, New York, 1961, Chapter 10.
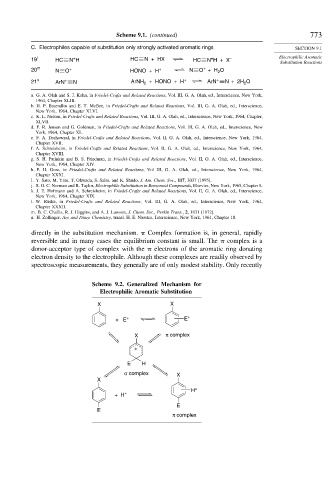
directly in the substitution mechanism. Complex formation is, in general, rapidly
reversible and in many cases the equilibrium constant is small. The complex is a
donor-acceptor type of complex with the electrons of the aromatic ring donating
electron density to the electrophile. Although these complexes are readily observed by
spectroscopic measurements, they generally are of only modest stability. Only recently
Scheme 9.2. Generalized Mechanism for
Electrophilic Aromatic Substitution
X X
+ E + E +
X π complex
+
E H
σ complex X
X
H +
+ H +
E
E
π complex