Page 795 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 795
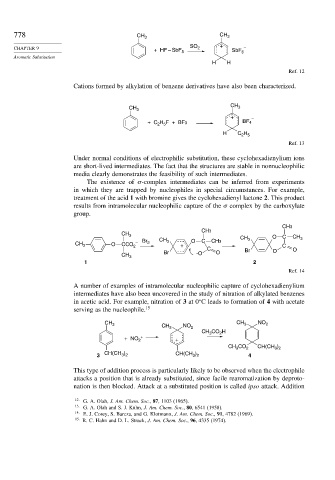
778 CH 3 CH 3
SO +
CHAPTER 9 + HF – SbF 5 2 SbF 6 –
Aromatic Substitution
H H
Ref. 12
Cations formed by alkylation of benzene derivatives have also been characterized.
CH
CH 3 3
+ –
+ C H F + BF3 BF 4
2 5
H C H
2 5
Ref. 13
Under normal conditions of electrophilic substitution, these cyclohexadienylium ions
are short-lived intermediates. The fact that the structures are stable in nonnucleophilic
media clearly demonstrates the feasibility of such intermediates.
The existence of -complex intermediates can be inferred from experiments
in which they are trapped by nucleophiles in special circumstances. For example,
treatment of the acid 1 with bromine gives the cyclohexadienyl lactone 2. This product
results from intramolecular nucleophilic capture of the complex by the carboxylate
group.
CH3
CH3
CH 3 CH O C CH 3
CH 3 O CCO 2 – Br 2 CH 3 + O C CH3 3 C
C Br O
Br O O
CH 3 -O
1 2
Ref. 14
A number of examples of intramolecular nucleophilic capture of cyclohexadienylium
intermediates have also been uncovered in the study of nitration of alkylated benzenes
in acetic acid. For example, nitration of 3 at 0 C leads to formation of 4 with acetate
serving as the nucleophile. 15
CH 3 CH NO CH 3 NO 2
2
3
CH CO H
2
3
+ NO 2 + +
CH CO 2 CH(CH )
3
3 2
CH(CH ) CH(CH )
3 3 2 3 2 4
This type of addition process is particularly likely to be observed when the electrophile
attacks a position that is already substituted, since facile rearomatization by deproto-
nation is then blocked. Attack at a substituted position is called ipso attack. Addition
12
G. A. Olah, J. Am. Chem. Soc., 87, 1103 (1965).
13 G. A. Olah and S. J. Kuhn, J. Am. Chem. Soc., 80, 6541 (1958).
14 E. J. Corey, S. Barcza, and G. Klotmann, J. Am. Chem. Soc., 91, 4782 (1969).
15
R. C. Hahn and D. L. Strack, J. Am. Chem. Soc., 96, 4335 (1974).