Page 799 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 799
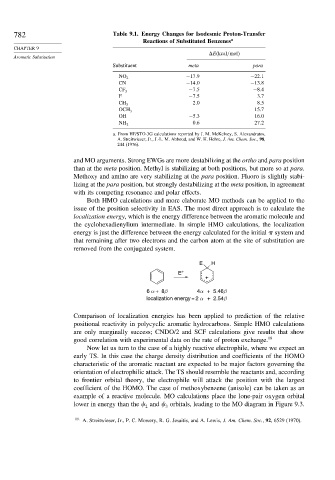
782 Table 9.1. Energy Changes for Isodesmic Proton-Transfer
Reactions of Substituted Benzenes a
CHAPTER 9
E kcal/mol
Aromatic Substitution
Substituent meta para
−17 9 −22 1
NO 2
CN −14 0 −13 8
−7 5 −8 4
CF 3
F −7 5 3 7
2 0 8 5
CH 3
15 7
OCH 3
OH −5 3 16 0
0 6 27 2
NH 2
a. From HF/STO-3G calculations reported by J. M. McKelvey, S. Alexandratos,
A. Streitwieser, Jr., J.-L. M. Abboud, and W. H. Hehre, J. Am. Chem. Soc., 98,
244 (1976).
and MO arguments. Strong EWGs are more destabilizing at the ortho and para position
than at the meta position. Methyl is stabilizing at both positions, but more so at para.
Methoxy and amino are very stabilizing at the para position. Fluoro is slightly stabi-
lizing at the para position, but strongly destabilizing at the meta position, in agreement
with its competing resonance and polar effects.
Both HMO calculations and more elaborate MO methods can be applied to the
issue of the position selectivity in EAS. The most direct approach is to calculate the
localization energy, which is the energy difference between the aromatic molecule and
the cyclohexadienylium intermediate. In simple HMO calculations, the localization
energy is just the difference between the energy calculated for the initial system and
that remaining after two electrons and the carbon atom at the site of substitution are
removed from the conjugated system.
E H
E +
+
6 α + 8β 4α + 5.46β
localization energy = 2 α + 2.54β
Comparison of localization energies has been applied to prediction of the relative
positional reactivity in polycyclic aromatic hydrocarbons. Simple HMO calculations
are only marginally success; CNDO/2 and SCF calculations give results that show
good correlation with experimental data on the rate of proton exchange. 19
Now let us turn to the case of a highly reactive electrophile, where we expect an
early TS. In this case the charge density distribution and coefficients of the HOMO
characteristic of the aromatic reactant are expected to be major factors governing the
orientation of electrophilic attack. The TS should resemble the reactants and, according
to frontier orbital theory, the electrophile will attack the position with the largest
coefficient of the HOMO. The case of methoxybenzene (anisole) can be taken as an
example of a reactive molecule. MO calculations place the lone-pair oxygen orbital
lower in energy than the and orbitals, leading to the MO diagram in Figure 9.3.
2 3
19
A. Streitwieser, Jr., P. C. Mowery, R. G. Jesaitis, and A. Lewis, J. Am. Chem. Soc., 92, 6529 (1970).