Page 796 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 796
products have also been isolated when initial electrophilic attack has occurred at an 779
unsubstituted position. The extent of addition in competition with substitution increases
on going to naphthalene and the larger polycyclic aromatic ring systems. 16 SECTION 9.2
The general mechanistic framework outlined in this section can be elaborated Structure-Reactivity
Relationships for
by other details to more fully describe the mechanisms of the individual electrophilic Substituted Benzenes
substitutions. The question of the identity of the active electrophile in each reaction is
important. We have discussed the case of nitration in which, under many circumstances,
the electrophile is the nitronium ion. Similar questions about the structure of the active
electrophile arise in most of the other substitution processes. Another issue that is
important is the ability of the electrophile to select among the alternative positions on
a substituted aromatic ring (position selectivity). The relative reactivity and selectivity
of substituted benzenes toward various electrophiles is important in developing a firm
understanding of EAS. The next section considers some of the structure-reactivity
relationships that have proven to be informative.
9.2. Structure-Reactivity Relationships for Substituted Benzenes
9.2.1. Substituent Effects on Reactivity
The effect that existing substituents have on EAS reactions has been studied since
about 1870. The classification of substituents as activating and ortho-para directing
or deactivating and meta directing became clear from these early studies. An under-
standing of the origin of these substituent effects became possible when ideas about
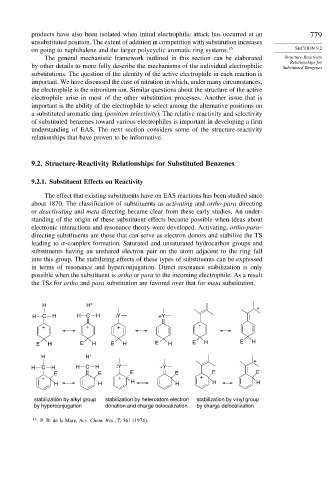
electronic interactions and resonance theory were developed. Activating, ortho-para–
directing substituents are those that can serve as electron donors and stabilize the TS
leading to -complex formation. Saturated and unsaturated hydrocarbon groups and
substituents having an unshared electron pair on the atom adjacent to the ring fall
into this group. The stabilizing effects of these types of substituents can be expressed
in terms of resonance and hyperconjugation. Direct resonance stabilization is only
possible when the substituent is ortho or para to the incoming electrophile. As a result
the TSs for ortho and para substitution are favored over that for meta substitution.
H H +
+
H C H H C H :Y +Y
+ + + +
E H E H E H E H E H E H
H H +
+
H C H H C H :Y +Y
E E E E E E
+ + +
H H H H H H
stabilization by alkyl group stabilization by heteroatom electron stabilization by vinyl group
by hyperconjugation donation and charge delocalization by charge delocalization
16
P. B. de la Mare, Acc. Chem. Res., 7, 361 (1974).