Page 797 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 797
780 As the substituent groups have a direct resonance interaction with the charge that
develops in the complex, quantitative substituent effects exhibit a high-resonance
CHAPTER 9 component. Hammett equations usually correlate best with the -substituent constants
+
Aromatic Substitution (see Section 3.5). 17
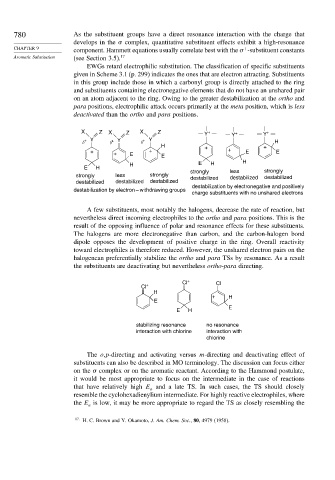
EWGs retard electrophilic substitution. The classification of specific substituents
given in Scheme 3.1 (p. 299) indicates the ones that are electron attracting. Substituents
in this group include those in which a carbonyl group is directly attached to the ring
and substituents containing electronegative elements that do not have an unshared pair
on an atom adjacent to the ring. Owing to the greater destabilization at the ortho and
para positions, electrophilic attack occurs primarily at the meta position, which is less
deactivated than the ortho and para positions.
X Z X Z X Z Y + Y + Y +
δ + Y δ + Y δ + Y H
H + +
+ + E + E + E E
H E H H
E H
strongly less strongly
strongly less strongly destabilized destabilized destabilized
destabilized destabilized destabilized
destabilization by electronegative and positively
destabilization by electron – withdrawing groups
charge substituents with no unshared electrons
A few substituents, most notably the halogens, decrease the rate of reaction, but
nevertheless direct incoming electrophiles to the ortho and para positions. This is the
result of the opposing influence of polar and resonance effects for these substituents.
The halogens are more electronegative than carbon, and the carbon-halogen bond
dipole opposes the development of positive charge in the ring. Overall reactivity
toward electrophiles is therefore reduced. However, the unshared electron pairs on the
halogencan preferentially stabilize the ortho and para TSs by resonance. As a result
the substituents are deactivating but nevertheless ortho-para directing.
Cl + Cl
Cl +
H
+ H
E
E
E H
stabilizing resonance no resonance
interaction with chlorine interaction with
chlorine
The o,p-directing and activating versus m-directing and deactivating effect of
substituents can also be described in MO terminology. The discussion can focus either
on the complex or on the aromatic reactant. According to the Hammond postulate,
it would be most appropriate to focus on the intermediate in the case of reactions
that have relatively high E and a late TS. In such cases, the TS should closely
a
resemble the cyclohexadienylium intermediate. For highly reactive electrophiles, where
the E is low, it may be more appropriate to regard the TS as closely resembling the
a
17
H. C. Brown and Y. Okamoto, J. Am. Chem. Soc., 80, 4979 (1958).