Page 798 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 798
reactant aromatic. Let us examine the MO description of substituent effects from both 781
these perspectives.
The TS resembles the intermediate, a substituted cyclohexadienylium ion. The SECTION 9.2
electrophile has localized one pair of electrons to form the new bond. The Structure-Reactivity
Relationships for
Hückel orbitals are the same as for the pentadienyl system, as shown in Figure 9.2. Substituted Benzenes
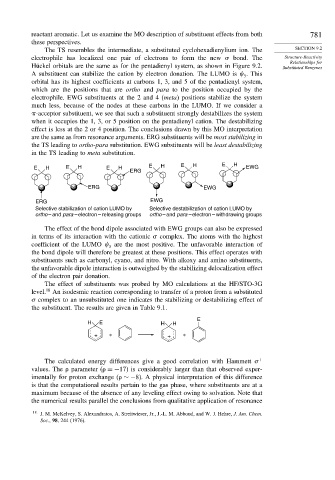
A substituent can stabilize the cation by electron donation. The LUMO is . This
3
orbital has its highest coefficients at carbons 1, 3, and 5 of the pentadienyl system,
which are the positions that are ortho and para to the position occupied by the
electrophile. EWG substituents at the 2 and 4 (meta) positions stabilize the system
much less, because of the nodes at these carbons in the LUMO. If we consider a
-acceptor substituent, we see that such a substituent strongly destabilizes the system
when it occupies the 1, 3, or 5 position on the pentadienyl cation. The destabilizing
effect is less at the 2 or 4 position. The conclusions drawn by this MO interpretation
are the same as from resonance arguments. ERG substituents will be most stabilizing in
the TS leading to ortho-para substitution. EWG substituents will be least destabilizing
in the TS leading to meta substitution.
E H E H E H E H E H E H EWG
ERG
ERG EWG
ERG EWG
Selective stabilization of cation LUMO by Selective destabilization of cation LUMO by
ortho – and para – electron – releasing groups ortho – and para – electron – withdrawing groups
The effect of the bond dipole associated with EWG groups can also be expressed
in terms of its interaction with the cationic complex. The atoms with the highest
coefficient of the LUMO are the most positive. The unfavorable interaction of
3
the bond dipole will therefore be greatest at these positions. This effect operates with
substituents such as carbonyl, cyano, and nitro. With alkoxy and amino substituents,
the unfavorable dipole interaction is outweighed by the stabilizing delocalization effect
of the electron pair donation.
The effect of substituents was probed by MO calculations at the HF/STO-3G
18
level. An isodesmic reaction corresponding to transfer of a proton from a substituted
complex to an unsubstituted one indicates the stabilizing or destabilizing effect of
the substituent. The results are given in Table 9.1.
E
H E H H
+ + + +
The calculated energy differences give a good correlation with Hammett +
values. The parameter ( =−17) is considerably larger than that observed exper-
imentally for proton exchange ( ∼−8). A physical interpretation of this difference
is that the computational results pertain to the gas phase, where substituents are at a
maximum because of the absence of any leveling effect owing to solvation. Note that
the numerical results parallel the conclusions from qualitative application of resonance
18
J. M. McKelvey, S. Alexandratos, A. Streitwieser, Jr., J.-L. M. Abboud, and W. J. Hehre, J. Am. Chem.
Soc., 98, 244 (1976).