Page 800 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 800
783
SECTION 9.2
Structure-Reactivity
Relationships for
Substituted Benzenes
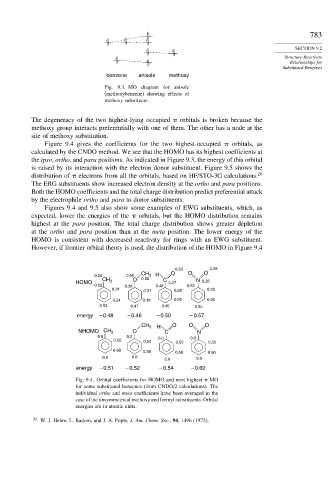
benzene anisole methoxy
Fig. 9.3. MO diagram for anisole
(methoxybenzene) showing effects of
methoxy substituent.
The degeneracy of the two highest-lying occupied orbitals is broken because the
methoxy group interacts preferentially with one of them. The other has a node at the
site of methoxy substitution.
Figure 9.4 gives the coefficients for the two highest-occupied orbitals, as
calculated by the CNDO method. We see that the HOMO has its highest coefficients at
the ipso, ortho, and para positions. As indicated in Figure 9.3, the energy of this orbital
is raised by its interaction with the electron donor substituent. Figure 9.5 shows the
distribution of electrons from all the orbitals, based on HF/STO-3G calculations. 20
The ERG substituents show increased electron density at the ortho and para positions.
Both the HOMO coefficients and the total charge distribution predict preferential attack
by the electrophile ortho and para to donor substituents.
Figures 9.4 and 9.5 also show some examples of EWG substituents, which, as
expected, lower the energies of the orbitals, but the HOMO distribution remains
highest at the para position. The total charge distribution shows greater depletion
at the ortho and para position than at the meta position. The lower energy of the
HOMO is consistent with decreased reactivity for rings with an EWG substituent.
However, if frontier orbital theory is used, the distribution of the HOMO in Figure 9.4
0.53 0.28
0.20 0.56 CH 3 H O O O
CH O 0.58 C
HOMO 3 0.27 N 0.26
0.52 0.38 0.46 0.51
0.31 0.31 0.28 0.23
0.24 0.18 0.20 0.26
0.53 0.47 0.45 0.50
energy – 0.48 – 0.46 – 0.50 – 0.57
CH 3 H O O O
NHOMO CH 3 O C N
0.0 0.0 0.0 0.0
0.50 0.50 0.50 0.50
0.50 0.50 0.50 0.50
0.0 0.0 0.0
0.0
energy – 0.51 – 0.52 – 0.54 – 0.60
Fig. 9.4. Orbital coefficients for HOMO and next highest MO
for some substituted benzenes (from CNDO/2 calculations). The
individual ortho and meta coefficients have been averaged in the
case of the unsymmetrical methoxy and formyl substituents. Orbital
energies are in atomic units.
20
W. J. Hehre, L. Radom, and J. A. Pople, J. Am. Chem. Soc., 94, 1496 (1972).