Page 805 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 805
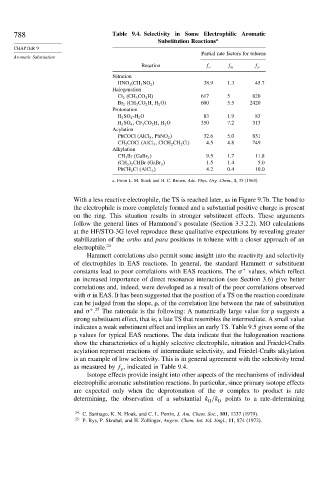
788 Table 9.4. Selectivity in Some Electrophilic Aromatic
Substitution Reactions a
CHAPTER 9
Partial rate factors for toluene
Aromatic Substitution
Reaction f o f m f p
Nitration
HNO 3 (CH 3 NO 2 38 9 1 3 45 7
Halogenation
Cl 2 (CH 3 CO 2 H) 617 5 820
Br 2 (CH 3 CO 2 H, H 2 O) 600 5 5 2420
Protonation
H 2 SO 4 -H 2 O 83 1 9 83
H 2 SO 4 ,CF 3 CO 2 H, H 2 O 350 7 2 313
Acylation
PhCOCl (AlCl 3 , PhNO 2 32 6 5 0 831
CH 3 COCl (AlCl 3 , ClCH 2 CH 2 Cl) 4 5 4 8 749
Alkylation
CH 3 Br (GaBr 3 9 5 1 7 11 8
(CH 3 2 CHBr (GaBr 3 1 5 1 4 5 0
PhCH 2 Cl (AlCl 3 4 2 0 4 10 0
a. From L. M. Stock and H. C. Brown, Adv. Phys. Org. Chem., 1, 35 (1963).
With a less reactive electrophile, the TS is reached later, as in Figure 9.7b. The bond to
the electrophile is more completely formed and a substantial positive charge is present
on the ring. This situation results in stronger substituent effects. These arguments
follow the general lines of Hammond’s postulate (Section 3.3.2.2). MO calculations
at the HF/STO-3G level reproduce these qualitative expectations by revealing greater
stabilization of the ortho and para positions in toluene with a closer approach of an
electrophile. 24
Hammett correlations also permit some insight into the reactivity and selectivity
of electrophiles in EAS reactions. In general, the standard Hammett substituent
+
constants lead to poor correlations with EAS reactions. The values, which reflect
an increased importance of direct resonance interaction (see Section 3.6) give better
correlations and, indeed, were developed as a result of the poor correlations observed
with in EAS. It has been suggested that the position of a TS on the reaction coordinate
can be judged from the slope, , of the correlation line between the rate of substitution
+ 25
and . The rationale is the following: A numerically large value for suggests a
strong substituent effect, that is, a late TS that resembles the intermediate. A small value
indicates a weak substituent effect and implies an early TS. Table 9.5 gives some of the
values for typical EAS reactions. The data indicate that the halogenation reactions
show the characteristics of a highly selective electrophile, nitration and Friedel-Crafts
acylation represent reactions of intermediate selectivity, and Friedel-Crafts alkylation
is an example of low selectivity. This is in general agreement with the selectivity trend
as measured by f , indicated in Table 9.4.
p
Isotope effects provide insight into other aspects of the mechanisms of individual
electrophilic aromatic substitution reactions. In particular, since primary isotope effects
are expected only when the deprotonation of the complex to product is rate
determining, the observation of a substantial k /k D points to a rate-determining
H
24 C. Santiago, K. N. Houk, and C. L. Perrin, J. Am. Chem. Soc., 101, 1337 (1979).
25
P. Rys, P. Skrabal, and H. Zollinger, Angew. Chem. Int. Ed. Engl., 11, 874 (1972).