Page 806 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 806
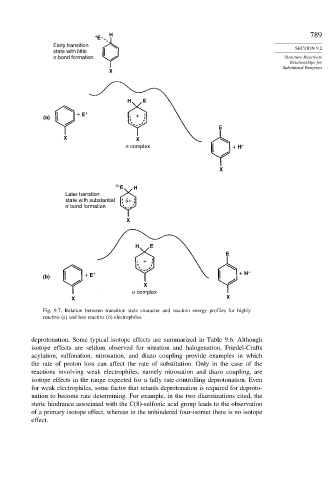
+ E H 789
Early transition SECTION 9.2
state with little
σ bond formation Structure-Reactivity
Relationships for
Substituted Benzenes
X
H E
+ E + +
(a)
E
X X
σ complex + H +
X
δ+ E H
Later transtion
state with substantial δ+
σ bond formation
X
H E
E
+
(b) + E + + H +
X
σ complex
X X
Fig. 9.7. Relation between transition state character and reaction energy profiles for highly
reactive (a) and less reactive (b) electrophiles.
deprotonation. Some typical isotope effects are summarized in Table 9.6. Although
isotope effects are seldom observed for nitration and halogenation, Friedel-Crafts
acylation, sulfonation, nitrosation, and diazo coupling provide examples in which
the rate of proton loss can affect the rate of substitution. Only in the case of the
reactions involving weak electrophiles, namely nitrosation and diazo coupling, are
isotope effects in the range expected for a fully rate-controlling deprotonation. Even
for weak electrophiles, some factor that retards deprotonation is required for deproto-
nation to become rate determining. For example, in the two diazotizations cited, the
steric hindrance associated with the C(8)-sulfonic acid group leads to the observation
of a primary isotope effect, whereas in the unhindered four-isomer there is no isotope
effect.