Page 808 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 808
E H 791
E + E +
+ E H SECTION 9.3
Reactivity of Polycyclic
+
and Heteroaromatic
reactants Compounds
reactants
products products
(a) rate-controlling formation of the (b) rate-controlling σ complex
electrophile formation (non selective electrophile)
E H
E H
+
E + E +
+
reactants
reactants
products products
(c) rate-controlling σ complex (d) rate-controlling deprotonation
formation (selective electrophile)
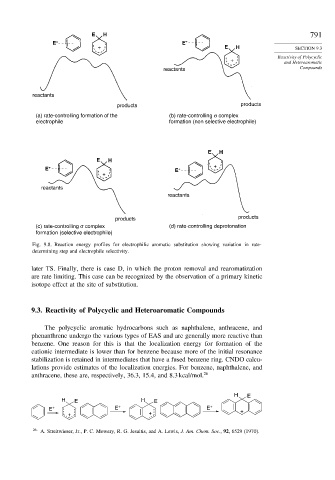
Fig. 9.8. Reaction energy profiles for electrophilic aromatic substitution showing variation in rate-
determining step and electrophile selectivity.
later TS. Finally, there is case D, in which the proton removal and rearomatization
are rate limiting. This case can be recognized by the observation of a primary kinetic
isotope effect at the site of substitution.
9.3. Reactivity of Polycyclic and Heteroaromatic Compounds
The polycyclic aromatic hydrocarbons such as naphthalene, anthracene, and
phenanthrene undergo the various types of EAS and are generally more reactive than
benzene. One reason for this is that the localization energy for formation of the
cationic intermediate is lower than for benzene because more of the initial resonance
stabilization is retained in intermediates that have a fused benzene ring. CNDO calcu-
lations provide estimates of the localization energies. For benzene, naphthalene, and
anthracene, these are, respectively, 36.3, 15.4, and 8.3 kcal/mol. 26
H E
H E H E
E + E + E + +
+ +
26 A. Streitwieser, Jr., P. C. Mowery, R. G. Jesaitis, and A. Lewis, J. Am. Chem. Soc., 92, 6529 (1970).