Page 812 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 812
the LUMO and HOMO,
= − /2. 34 Thus the harder a reactant ring 795
LUMO HOMO
system is, the more difficult it is for an electrophile to complete -bond formation.
SECTION 9.3
E + E + Reactivity of Polycyclic
and Heteroaromatic
Compounds
more difficult less difficult
larger E a smaller E a
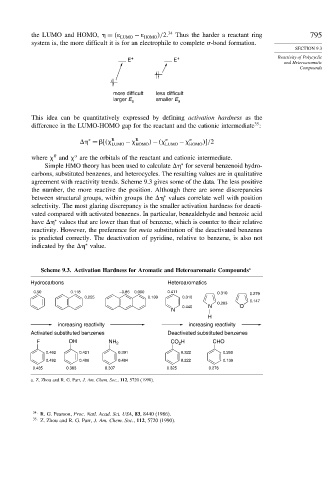
This idea can be quantitatively expressed by defining activation hardness as the
35
difference in the LUMO-HOMO gap for the reactant and the cationic intermediate :
∗
= R LUMO − R HOMO − LUMO − HOMO /2
R
where and are the orbitals of the reactant and cationic intermediate.
Simple HMO theory has been used to calculate
for several benzenoid hydro-
∗
carbons, substituted benzenes, and heterocycles. The resulting values are in qualitative
agreement with reactivity trends. Scheme 9.3 gives some of the data. The less positive
the number, the more reactive the position. Although there are some discrepancies
between structural groups, within groups the
values correlate well with position
∗
selectivity. The most glaring discrepancy is the smaller activation hardness for deacti-
vated compared with activated benzenes. In particular, benzaldehyde and benzoic acid
∗
have
values that are lower than that of benzene, which is counter to their relative
reactivity. However, the preference for meta substitution of the deactivated benzenes
is predicted correctly. The deactivation of pyridine, relative to benzene, is also not
∗
indicated by the
value.
Scheme 9.3. Activation Hardness for Aromatic and Heteroaromatic Compounds a
Hydrocarbons Heteroaromatics
0.50 0.118 –0.86 0.090 0.411 0.310 0.279
0.255 0.139 0.310
0.147
0.203
0.440 N O
N
H
increasing reactivity increasing reactivity
Activated substituted benzenes Deactivated substituted benzenes
F OH NH 2 CO H CHO
2
0.462 0.421 0.391 0.322 0.269
0.492 0.486 0.484 0.222 0.139
0.435 0.363 0.307 0.325 0.276
a. Z. Zhou and R. G. Parr, J. Am. Chem. Soc., 112, 5720 (1990).
34 R. G. Pearson, Proc. Natl. Acad. Sci. USA, 83, 8440 (1986).
35
Z. Zhou and R. G. Parr, J. Am. Chem. Soc., 112, 5720 (1990).