Page 816 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 816
Table 9.7. When the aromatic reactant carries an EWG, the selectivity increases, since 799
the TS occurs later. For example, while toluene is about 20 times more reactive than
benzene, p-nitrotoluene is about 200 times more reactive than nitrobenzene. The effect SECTION 9.4
of the methyl substituent is magnified as a result of the later TS. Specific Electrophilic
Substitution Reactions
An aspect of aromatic nitration that has received attention is the role of
charge transfer complexes and electron transfer intermediates on the path to the -
complex intermediate. For some NO -X nitrating reagents, the mechanism may involve
2
formation of a distinct electron transfer intermediate prior to the formation of the
complex. 45
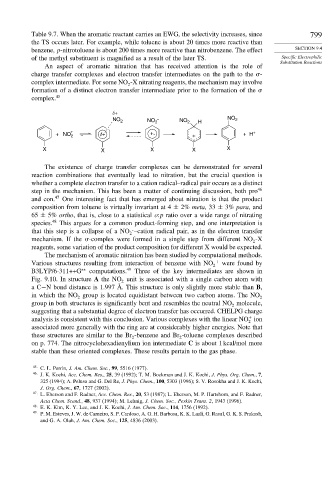
δ+
NO 2 NO 2 . NO 2 H NO 2
+ +
+ NO 2 δ+ +. + + H
X X X X X
The existence of charge transfer complexes can be demonstrated for several
reaction combinations that eventually lead to nitration, but the crucial question is
whether a complete electron transfer to a cation radical–radical pair occurs as a distinct
step in the mechanism. This has been a matter of continuing discussion, both pro 46
and con. 47 One interesting fact that has emerged about nitration is that the product
composition from toluene is virtually invariant at 4 ± 2% meta,33 ± 3% para, and
65 ± 5% ortho, that is, close to a statistical o:p ratio over a wide range of nitrating
species. 48 This argues for a common product-forming step, and one interpretation is
.
that this step is a collapse of a NO –cation radical pair, as in the electron transfer
2
mechanism. If the -complex were formed in a single step from different NO -X
2
reagents, some variation of the product composition for different X would be expected.
The mechanism of aromatic nitration has been studied by computational methods.
Various structures resulting from interaction of benzene with NO 2 + were found by
B3LYP/6-311++G ∗∗ computations. 49 Three of the key intermediates are shown in
Fig. 9.10. In structure A the NO unit is associated with a single carbon atom with
2
aC−N bond distance is 1.997 Å. This structure is only slightly more stable than B,
in which the NO group is located equidistant between two carbon atoms. The NO 2
2
group in both structures is significantly bent and resembles the neutral NO molecule,
2
suggesting that a substantial degree of electron transfer has occurred. CHELPG charge
+
analysis is consistent with this conclusion. Various complexes with the linear NO ion
2
associated more generally with the ring are at considerably higher energies. Note that
these structures are similar to the Br -benzene and Br -toluene complexes described
2
2
on p. 774. The nitrocyclohexadienylium ion intermediate C is about 1 kcal/mol more
stable than these oriented complexes. These results pertain to the gas phase.
45 C. L. Perrin, J. Am. Chem. Soc., 99, 5516 (1977).
46 J. K. Kochi, Acc. Chem. Res., 25, 39 (1992); T. M. Bockman and J. K. Kochi, J. Phys. Org. Chem., 7,
325 (1994); A. Peluso and G. Del Re, J. Phys. Chem., 100, 5303 (1996); S. V. Rosokha and J. K. Kochi,
J. Org. Chem., 67, 1727 (2002).
47
L. Eberson and F. Radner, Acc. Chem. Res., 20, 53 (1987); L. Eberson, M. P. Hartshorn, and F. Radner,
Acta Chem. Scand., 48, 937 (1994); M. Lehnig, J. Chem. Soc., Perkin Trans. 2, 1943 (1996).
48 E. K. Kim, K. Y. Lee, and J. K. Kochi, J. Am. Chem. Soc., 114, 1756 (1992).
49
P. M. Esteves, J. W. de Carneiro, S. P. Cardoso, A. G. H. Barbosa, K. K. Laali, G. Rasul, G. K. S. Prakash,
and G. A. Olah, J. Am. Chem. Soc., 125, 4836 (2003).