Page 813 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 813
796 9.4. Specific Electrophilic Substitution Reactions
CHAPTER 9
At this point, we focus on specific electrophilic substitution reactions. The kinds
Aromatic Substitution
of data that have been especially pertinent in elucidating mechanistic detail include
linear free-energy relationships, kinetic studies, isotope effects, and selectivity patterns.
In general, the basic questions to be asked about each mechanism are: (1) What is
the active electrophile? (2) Which step in the general mechanism for EAS is rate
determining? (3) What are the orientation and selectivity patterns?
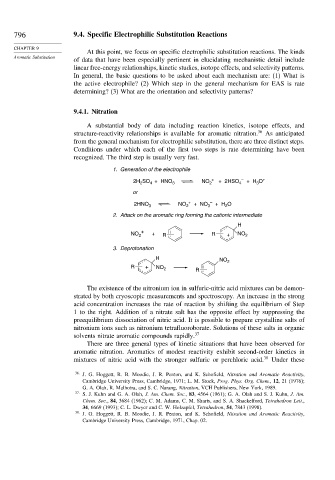
9.4.1. Nitration
A substantial body of data including reaction kinetics, isotope effects, and
structure-reactivity relationships is available for aromatic nitration. 36 As anticipated
from the general mechanism for electrophilic substitution, there are three distinct steps.
Conditions under which each of the first two steps is rate determining have been
recognized. The third step is usually very fast.
1. Generation of the electrophile
+
–
2H SO + HNO 3 NO + 2HSO + H O +
4
3
4
2
2
or
–
+
2HNO 3 NO + NO + H O
2
2
3
2. Attack on the aromatic ring forming the cationic intermediate
H
NO 2 + + R R + NO 2
3. Deprotonation
H NO 2
R + NO 2
R
The existence of the nitronium ion in sulfuric-nitric acid mixtures can be demon-
strated by both cryoscopic measurements and spectroscopy. An increase in the strong
acid concentration increases the rate of reaction by shifting the equilibrium of Step
1 to the right. Addition of a nitrate salt has the opposite effect by suppressing the
preequilibrium dissociation of nitric acid. It is possible to prepare crystalline salts of
nitronium ions such as nitronium tetrafluoroborate. Solutions of these salts in organic
solvents nitrate aromatic compounds rapidly. 37
There are three general types of kinetic situations that have been observed for
aromatic nitration. Aromatics of modest reactivity exhibit second-order kinetics in
mixtures of nitric acid with the stronger sulfuric or perchloric acid. 38 Under these
36
J. G. Hoggett, R. B. Moodie, J. R. Penton, and K. Schofield, Nitration and Aromatic Reactivity,
Cambridge University Press, Cambridge, 1971; L. M. Stock, Prog. Phys. Org. Chem., 12, 21 (1976);
G. A. Olah, R. Malhotra, and S. C. Narang, Nitration, VCH Publishers, New York, 1989.
37 S. J. Kuhn and G. A. Olah, J. Am. Chem. Soc., 83, 4564 (1961); G. A. Olah and S. J. Kuhn, J. Am.
Chem. Soc., 84, 3684 (1962); C. M. Adams, C. M. Sharts, and S. A. Shackelford, Tetrahedron Lett.,
34, 6669 (1993); C. L. Dwyer and C. W. Holzapfel, Tetrahedron, 54, 7843 (1998).
38
J. G. Hoggett, R. B. Moodie, J. R. Penton, and K. Schofield, Nitration and Aromatic Reactivity,
Cambridge University Press, Cambridge, 1971, Chap. 02.