Page 809 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 809
792 The relative stability of the TSs determines the position of substitution under
kinetically controlled conditions. For naphthalene, the preferred site for electrophilic
CHAPTER 9 attack is the 1-position, which is the result of the greater stability of the cationic
Aromatic Substitution intermediate for 1-substitution.
H E E
E + E + + H
+
cationic intermediate cationic intermediate
has both allylic and has benzylic but not
benzylic stabilization allylic stabilization
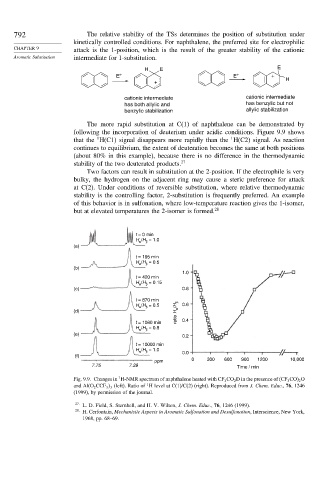
The more rapid substitution at C(1) of naphthalene can be demonstrated by
following the incorporation of deuterium under acidic conditions. Figure 9.9 shows
1
1
that the H(C1) signal disappears more rapidly than the H(C2) signal. As reaction
continues to equilibrium, the extent of deuteration becomes the same at both positions
(about 80% in this example), because there is no difference in the thermodynamic
stability of the two deuterated products. 27
Two factors can result in substitution at the 2-position. If the electrophile is very
bulky, the hydrogen on the adjacent ring may cause a steric preference for attack
at C(2). Under conditions of reversible substitution, where relative thermodynamic
stability is the controlling factor, 2-substitution is frequently preferred. An example
of this behavior is in sulfonation, where low-temperature reaction gives the 1-isomer,
but at elevated temperatures the 2-isomer is formed. 28
t = 0 min
β
H α /H = 1.0
(a)
t = 195 min
H /H = 0.5
β
α
(b)
1.0
t = 420 min
H α /H = 0.15
β
(c) 0.8
t = 870 min
β
H α /H = 0.5 0.6
(d) ratio H α /H β
t = 1080 min 0.4
H α /H = 0.8
β
(e) 0.2
t = 10000 min
β
H α /H = 1.0 0.0
(f)
ppm 0 300 600 900 1200 10,000
7.75 7.39
Time / min
1
Fig. 9.9. Changes in H-NMR spectrum of naphthalene heated with CF 3 CO 2 D in the presence of (CF 3 CO) 2 O
1
and Al(O 2 CCF 3 3 (left). Ratio of H level at C(1)/C(2) (right). Reproduced from J. Chem. Educ., 76, 1246
(1999), by permission of the journal.
27 L. D. Field, S. Sternhell, and H. V. Wilton, J. Chem. Educ., 76, 1246 (1999).
28
H. Cerfontain, Mechanistic Aspects in Aromatic Sulfonation and Desulfonation, Interscience, New York,
1968, pp. 68–69.