Page 811 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 811
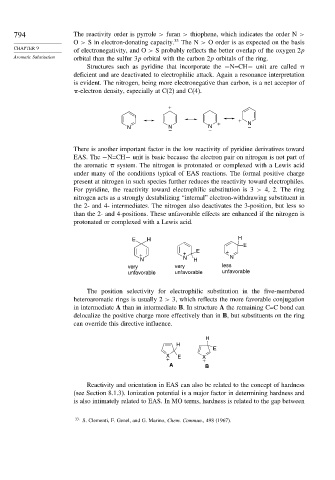
794 The reactivity order is pyrrole > furan > thiophene, which indicates the order N >
O > S in electron-donating capacity. 33 The N > O order is as expected on the basis
CHAPTER 9
of electronegativity, and O > S probably reflects the better overlap of the oxygen 2p
Aromatic Substitution orbital than the sulfur 3p orbital with the carbon 2p orbitals of the ring.
Structures such as pyridine that incorporate the −N=CH− unit are called
deficient and are deactivated to electrophilic attack. Again a resonance interpretation
is evident. The nitrogen, being more electronegative than carbon, is a net acceptor of
-electron density, especially at C(2) and C(4).
+
+
+ N
N N N –
– –
There is another important factor in the low reactivity of pyridine derivatives toward
EAS. The −N=CH− unit is basic because the electron pair on nitrogen is not part of
the aromatic system. The nitrogen is protonated or complexed with a Lewis acid
under many of the conditions typical of EAS reactions. The formal positive charge
present at nitrogen in such species further reduces the reactivity toward electrophiles.
For pyridine, the reactivity toward electrophilic substitution is 3 > 4, 2. The ring
nitrogen acts as a strongly destabilizing “internal” electron-withdrawing substituent in
the 2- and 4- intermediates. The nitrogen also deactivates the 3-position, but less so
than the 2- and 4-positions. These unfavorable effects are enhanced if the nitrogen is
protonated or complexed with a Lewis acid.
E H H
E
E +
+ + N
N N H
very very less
unfavorable unfavorable unfavorable
The position selectivity for electrophilic substitution in the five-membered
heteroaromatic rings is usually 2 > 3, which reflects the more favorable conjugation
in intermediate A than in intermediate B. In structure A the remaining C=C bond can
delocalize the positive charge more effectively than in B, but substituents on the ring
can override this directive influence.
H
H
E
X E X
+ +
A B
Reactivity and orientation in EAS can also be related to the concept of hardness
(see Section 8.1.3). Ionization potential is a major factor in determining hardness and
is also intimately related to EAS. In MO terms, hardness is related to the gap between
33
S. Clementi, F. Genel, and G. Marino, Chem. Commun., 498 (1967).