Page 815 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 815
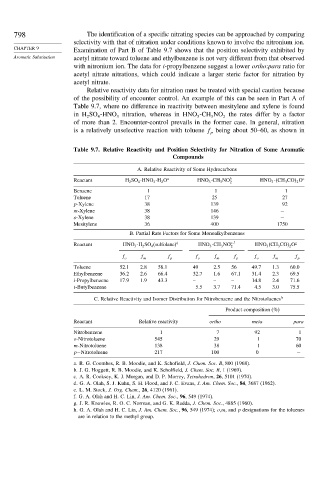
798 The identification of a specific nitrating species can be approached by comparing
selectivity with that of nitration under conditions known to involve the nitronium ion.
CHAPTER 9 Examination of Part B of Table 9.7 shows that the position selectivity exhibited by
Aromatic Substitution acetyl nitrate toward toluene and ethylbenzene is not very different from that observed
with nitronium ion. The data for i-propylbenzene suggest a lower ortho:para ratio for
acetyl nitrate nitrations, which could indicate a larger steric factor for nitration by
acetyl nitrate.
Relative reactivity data for nitration must be treated with special caution because
of the possibility of encounter control. An example of this can be seen in Part A of
Table 9.7, where no difference in reactivity between mesitylene and xylene is found
in H SO -HNO nitration, whereas in HNO -CH NO the rates differ by a factor
2 4 3 3 3 2
of more than 2. Encounter-control prevails in the former case. In general, nitration
is a relatively unselective reaction with toluene f being about 50–60, as shown in
p
Table 9.7. Relative Reactivity and Position Selectivity for Nitration of Some Aromatic
Compounds
A. Relative Reactivity of Some Hydrocarbons
Reactant H 2 SO 4 -HNO 3 -H 2 O a HNO 3 -CH 3 NO b 2 HNO 3 - CH 3 CO 2 O c
Benzene 1 1 1
Toluene 17 25 27
p-Xylene 38 139 92
m-Xylene 38 146 −
o-Xylene 38 139 −
Mesitylene 36 400 1750
B. Partial Rate Factors for Some Monoalkylbenzenes
Reactant HNO 3 -H 2 SO 4 (sulfolane) d HNO 3 -CH 3 NO e
f HNO 3 (CH 3 CO) 2 O g
2
f o f m f p f o f m f p f o f m f p
Toluene 52.1 2.8 58.1 49 2.5 56 49.7 1.3 60.0
Ethylbenzene 36.2 2.6 66.4 32.7 1.6 67.1 31.4 2.3 69.5
i-Propylbenzene 17.9 1.9 43.3 – – – 14.8 2.4 71.6
t-Butylbenzene 5.5 3.7 71.4 4.5 3.0 75.5
C. Relative Reactivity and Isomer Distribution for Nitrobenzene and the Nitrotoluenes h
Product composition (%)
Reactant Relative reactivity ortho meta para
Nitrobenzene 1 7 92 1
o-Nitrotoluene 545 29 1 70
m-Nitrotoluene 138 38 1 60
p−Nitrotoluene 217 100 0 −
a. R. G. Coombes, R. B. Moodie, and K. Schofield, J. Chem. Soc. B, 800 (1968).
b. J. G. Hoggett, R. B. Moodie, and K. Scholfield, J. Chem. Soc. B, 1 (1969).
c. A. R. Cooksey, K. J. Morgan, and D. P. Morrey, Tetrahedron, 26, 5101 (1970).
d. G. A. Olah, S. J. Kuhn, S. H. Flood, and J. C. Evans, J. Am. Chem. Soc., 84, 3687 (1962).
e. L. M. Stock, J. Org. Chem., 26, 4120 (1961).
f. G. A. Olah and H. C. Lin, J. Am. Chem. Soc., 96, 549 (1974).
g. J. R. Knowles, R. O. C. Norman, and G. K. Radda, J. Chem. Soc., 4885 (1960).
h. G. A. Olah and H. C. Lin, J. Am. Chem. Soc., 96, 549 (1974); o,m, and p designations for the toluenes
are in relation to the methyl group.