Page 819 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 819
+
802 Both Cl O and H OCl apparently are active electrophiles under these conditions.
2
2
The terms involving Cl O are zero order in the aromatic reactant because formation of
2
CHAPTER 9
Cl O is the rate-limiting step. Thermodynamic considerations argue strongly against
2
Aromatic Substitution rate-determining cleavage of H OCl to H O and Cl . The estimated equilibrium
+
+
2 2
+
constant for this dissociation is so small that the concentration of Cl would be far
too low to account for the observed reaction rate. 55
Molecular bromine is thought to be the reactive brominating agent in uncat-
alyzed brominations. The bromination of benzene and toluene are first order in both
56
bromine and the aromatic reactant in trifluoroacetic acid solution, but becomes more
57
complicated in the presence of water. The bromination of benzene in aqueous acetic
acid exhibits a first-order dependence on bromine concentration when bromide ion is
present. The observed rate is dependent on bromide ion concentration, decreasing with
increasing concentration. The acids presumably assist in the rate-determining step, as
in the case of chlorination. The detailed kinetics are consistent with a rate-determining
formation of the complex when bromide ion concentration is low, but with a shift
to reversible formation of the complex with rate-determining deprotonation at high
bromide ion concentration. 58
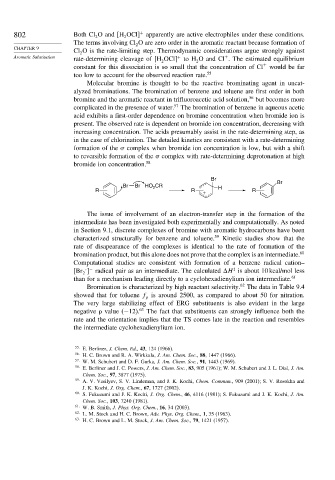
Br
Br Br HO CR H Br
2
R R R
+
The issue of involvement of an electron-transfer step in the formation of the
intermediate has been investigated both experimentally and computationally. As noted
in Section 9.1, discrete complexes of bromine with aromatic hydrocarbons have been
characterized structurally for benzene and toluene. 59 Kinetic studies show that the
rate of disapearance of the complexes is identical to the rate of formation of the
bromination product, but this alone does not prove that the complex is an intermediate. 60
Computational studies are consistent with formation of a benzene radical cation–
. −
Br radical pair as an intermediate. The calculated H is about 10 kcal/mol less
‡
2
than for a mechanism leading directly to a cyclohexadienylium ion intermediate. 61
62
Bromination is characterized by high reactant selectivity. The data in Table 9.4
showed that for toluene f is around 2500, as compared to about 50 for nitration.
p
The very large stabilizing effect of ERG substituents is also evident in the large
negative value (−12). 63 The fact that substituents can strongly influence both the
rate and the orientation implies that the TS comes late in the reaction and resembles
the intermediate cyclohexadienylium ion.
55 E. Berliner, J. Chem. Ed., 43, 124 (1966).
56
H. C. Brown and R. A. Wirkkala, J. Am. Chem. Soc., 88, 1447 (1966).
57
W. M. Schubert and D. F. Gurka, J. Am. Chem. Soc., 91, 1443 (1969).
58 E. Berliner and J. C. Powers, J. Am. Chem. Soc., 83, 905 (1961); W. M. Schubert and J. L. Dial, J. Am.
Chem. Soc., 97, 3877 (1975).
59
A. V. Vasilyev, S. V. Lindeman, and J. K. Kochi, Chem. Commun., 909 (2001); S. V. Rosokha and
J. K. Kochi, J. Org. Chem., 67, 1727 (2002).
60
S. Fukuzumi and J. K. Kochi, J. Org. Chem., 46, 4116 (1981); S. Fukuzumi and J. K. Kochi, J. Am.
Chem. Soc., 103, 7240 (1981).
61 W. B. Smith, J. Phys. Org. Chem., 16, 34 (2003).
62 L. M. Stock and H. C. Brown, Adv. Phys. Org. Chem., 1, 35 (1963).
63
H. C. Brown and L. M. Stock, J. Am. Chem. Soc., 79, 1421 (1957).