Page 817 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 817
800 136.7°
O
O 1.178
CHAPTER 9 O O
N N
1.185
Aromatic Substitution 1.187 1.175
1.997 H 2.331 2.331
H
H H H
H 1.085
1.426 1.442
H
H 1.447 H 1.387 H
1.415 1.376 H 1.404
H
A
B
1.213 O
H H 129.2°
1.415 1.474 N
O
H
1.587
1.115
H 1.371 H
H
C
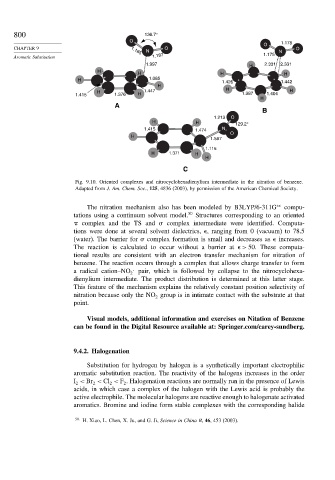
Fig. 9.10. Oriented complexes and nitrocyclohexadienylium intermediate in the nitration of benzene.
Adapted from J. Am. Chem. Soc., 125, 4836 (2003), by permission of the American Chemical Society.
The nitration mechanism also has been modeled by B3LYP/6-311G ∗∗ compu-
tations using a continuum solvent model. 50 Structures corresponding to an oriented
complex and the TS and complex intermediate were identified. Computa-
tions were done at several solvent dielectrics, , ranging from 0 (vacuum) to 78.5
(water). The barrier for complex formation is small and decreases as increases.
The reaction is calculated to occur without a barrier at > 50. These computa-
tional results are consistent with an electron transfer mechanism for nitration of
benzene. The reaction occurs through a complex that allows charge transfer to form
.
a radical cation–NO 2 pair, which is followed by collapse to the nitrocyclohexa-
dienylium intermediate. The product distribution is determined at this latter stage.
This feature of the mechanism explains the relatively constant position selectivity of
nitration because only the NO group is in intimate contact with the substrate at that
2
point.
Visual models, additional information and exercises on Nitation of Benzene
can be found in the Digital Resource available at: Springer.com/carey-sundberg.
9.4.2. Halogenation
Substitution for hydrogen by halogen is a synthetically important electrophilic
aromatic substitution reaction. The reactivity of the halogens increases in the order
I < Br < Cl < F . Halogenation reactions are normally run in the presence of Lewis
2
2
2
2
acids, in which case a complex of the halogen with the Lewis acid is probably the
active electrophile. The molecular halogens are reactive enough to halogenate activated
aromatics. Bromine and iodine form stable complexes with the corresponding halide
50
H. Xiao, L. Chen, X. Ju, and G. Ji, Science in China B, 46, 453 (2003).