Page 785 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 785
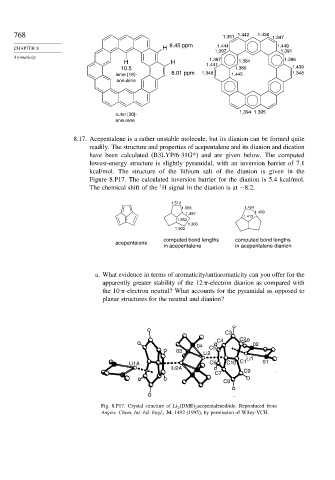
768 1.351 1.442 1.438 1.347
CHAPTER 8 H 8.45 ppm 1.444 1.449
1.397 1.391
Aromaticity 1.397 1.396
H H 1.441 1.384
10.5 1.385 1.439
inner [18]- 8.01 ppm 1.348 1.445 1.348
annulene
1.394 1.395
outer [30]-
annulene
8.17. Acepentalene is a rather unstable molecule, but its dianion can be formed quite
readily. The structure and properties of acepentalene and its dianion and dication
have been calculated (B3LYP/6-31G*) and are given below. The computed
lowest-energy structure is slightly pyramidal, with an inversion barrier of 7.1
kcal/mol. The structure of the lithium salt of the dianion is given in the
Figure 8.P17. The calculated inversion barrier for the dianion is 5.4 kcal/mol.
1
The chemical shift of the H signal in the dianion is at −8 2.
1.513
1.368 1.424
1.497 1.459
1.412
1.352
1.366
1.503
computed bond lengths computed bond lengths
acepentalene
in acepentalene in acepentalene dianion
a. What evidence in terms of aromaticity/antiaromaticity can you offer for the
apparently greater stability of the 12 -electron dianion as compared with
the 10 -electron neutral? What accounts for the pyramidal as opposed to
planar structures for the neutral and dianion?
C3
C4 C2
04 C5 02
03
Li2
Li1
Li1A C6 C10 C1 01
Li2A C9
C7
C8
Fig. 8.P17. Crystal structure of Li 2 (DME) 2 acepentalenediide. Reproduced from
Angew. Chem. Int. Ed. Engl., 34, 1492 (1995), by permission of Wiley-VCH.