Page 780 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 780
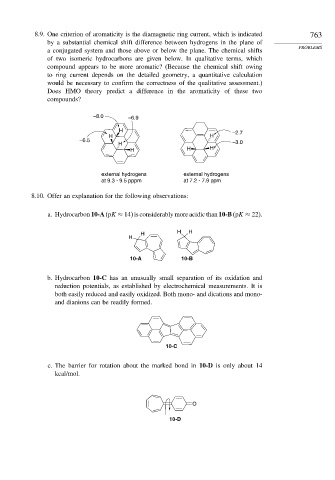
8.9. One criterion of aromaticity is the diamagnetic ring current, which is indicated 763
by a substantial chemical shift difference between hydrogens in the plane of
PROBLEMS
a conjugated system and those above or below the plane. The chemical shifts
of two isomeric hydrocarbons are given below. In qualitative terms, which
compound appears to be more aromatic? (Because the chemical shift owing
to ring current depends on the detailed geometry, a quantitative calculation
would be necessary to confirm the correctness of the qualitative assessment.)
Does HMO theory predict a difference in the aromaticity of these two
compounds?
–8.0 –6.9
H –2.7
H H
–6.5
H –3.0
H H H
external hydrogens external hydrogens
at 9.3 - 9.5 pppm at 7.2 - 7.9 ppm
8.10. Offer an explanation for the following observations:
a. Hydrocarbon 10-A (pK ≈ 14) is considerably more acidic than 10-B (pK ≈ 22).
H H
H
H
10-A 10-B
b. Hydrocarbon 10-C has an unusually small separation of its oxidation and
reduction potentials, as established by electrochemical measurements. It is
both easily reduced and easily oxidized. Both mono- and dications and mono-
and dianions can be readily formed.
10-C
c. The barrier for rotation about the marked bond in 10-D is only about 14
kcal/mol.
O
10-D