Page 778 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 778
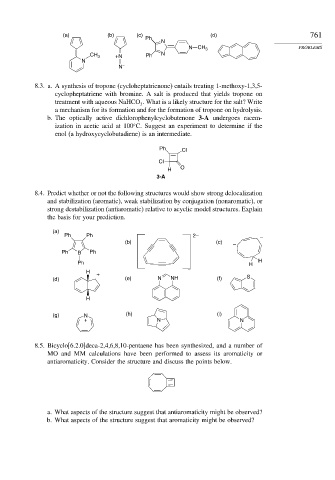
(a) (b) (c) (d) 761
Ph
N
N CH 3 PROBLEMS
CH 3 +N Ph N
N
N –
8.3. a. A synthesis of tropone (cycloheptatrienone) entails treating 1-methoxy-1,3,5-
cyclopheptatriene with bromine. A salt is produced that yields tropone on
treatment with aqueous NaHCO . What is a likely structure for the salt? Write
3
a mechanism for its formation and for the formation of tropone on hydrolysis.
b. The optically active dichlorophenylcyclobutenone 3-A undergoes racem-
ization in acetic acid at 100 C. Suggest an experiment to determine if the
enol (a hydroxycyclobutadiene) is an intermediate.
Ph Cl
Cl
H O
3-A
8.4. Predict whether or not the following structures would show strong delocalization
and stabilization (aromatic), weak stabilization by conjugation (nonaromatic), or
strong destabilization (antiaromatic) relative to acyclic model structures. Explain
the basis for your prediction.
(a)
Ph Ph 2– –
(b) (c) –
Ph B Ph
Ph H H
H
+
(d) (e) N NH (f) S
H
(g) N (h) (i)
+ N N
8.5. Bicyclo[6.2.0]deca-2,4,6,8,10-pentaene has been synthesized, and a number of
MO and MM calculations have been performed to assess its aromaticity or
antiaromaticity. Consider the structure and discuss the points below.
a. What aspects of the structure suggest that antiaromaticity might be observed?
b. What aspects of the structure suggest that aromaticity might be observed?